The Impact of Postoperative Prophylactic Medication on Long-Term Surgical, Severe Endoscopic and Endoscopic or Radiologic Recurrence Following Primary Ileocecal Resection in Patients With Crohn's Disease
- PMID: 39856782
- PMCID: PMC11869158
- DOI: 10.1111/apt.18496
The Impact of Postoperative Prophylactic Medication on Long-Term Surgical, Severe Endoscopic and Endoscopic or Radiologic Recurrence Following Primary Ileocecal Resection in Patients With Crohn's Disease
Abstract
Background: The impact of prophylactic medication following ileocecal resection (ICR) for Crohn's disease (CD) merits further elucidation. Prophylactic medication following ileocecal resection (ICR) is recommended in patients with Crohn's disease (CD), particularly in patients at increased risk of recurrence, but the impact on long-term outcomes needs to be further elucidated.
Aim: To evaluate the effect of postoperative prophylactic medication on long-term prognosis.
Methods: A retrospective cohort study was performed in patients with CD who underwent primary ICR between 2000-2020 in the Netherlands. Patients were divided into two groups: postoperative prophylactic medication [< 12 weeks following ICR] versus no postoperative prophylactic medication. Outcomes were surgical recurrence [re-resection for CD], severe endoscopic recurrence [modified Rutgeerts score (mRS) ≥ i3] and endoscopic or radiologic recurrence [mRS ≥ i2b or radiologic recurrence]. Inverse probability of treatment weighting [IPTW] method was used to adjust for confounding and selection bias. Survival and association between postoperative prophylactic medication and outcomes were assessed with Kaplan-Meier analyses and Cox proportional hazard models.
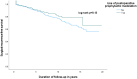
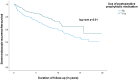
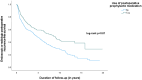
Results: 807 patients underwent ICR (median follow-up 5.0 years); 36% received postoperative prophylactic medication. Surgical, severe endoscopic and endoscopic or radiologic recurrence rates were significantly lower in those who received prophylactic medication (p = 0.01; p < 0.01; p < 0.01). IPTW analysis showed a lower risk of severe endoscopic and endoscopic or radiologic recurrence in patients treated with postoperative prophylactic medication (aOR 0.64; 95% CI 0.43-0.97; aOR 0.65; 95% CI 0.47-0.91), which also was identified as a protective factor for severe endoscopic (aHR 0.5; 95% CI 0.4-0.6) and endoscopic or radiologic recurrence (aHR 0.6, 95% CI 0.5-0.7) in multivariable analysis after correction for confounding factors. A comparable protective effect of postoperative prophylactic medication was sustained in patients who underwent ileocolonoscopy <1 year postoperatively and who underwent surgery on or after 2010.
Conclusions: Prophylactic medication following primary ICR significantly reduces long-term recurrence rates in CD and was identified as a protective factor for severe endoscopic and endoscopic or radiologic recurrence.
Keywords: Crohn's disease; intestinal surgery; postoperative recurrence; prophylactic medication.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
M.B. has received speaker fees from AbbVie and an unrestricted research grant from Falk Pharma BeNeLux, outside the submitted work. M.S. has received grants from AbbVie, Prometheus, Janssen, Takeda, and Pfizer; consulting fees from AbbVie, Amgen, Gilead, Janssen, Merck, Pfizer, and Takeda; honoraria from AbbVie, Amgen, Gilead, Janssen, Merck, Society for Continuing Professional Health Education (Canada), Takeda, and Pfizer; advisory board participation of AbbVie, Janssen, Pfizer, and Takeda, outside the submitted work. M.A. has served as a consultant/advisory board member to Abbvie, Amgen, Biogen, Boehringer‐Ingelheim, Bristol Myers Squibb, Celgene, Celsius, Celltrion, Egle Therapeutics, Endpoint health, Ferring, Galapagos, Genentech, IQVIA, Janssen, Lilly, Novartis, Pfizer, Roche, Takeda, Tillots; speaker for CME activities for Abbvie, Galapagos, Genentech, Janssen, Pfizer, Roche, Takeda, Tillots; grant support from Janssen, Takeda, Genentech/Roche, outside the submitted work. F.H. has served on advisory boards or as speaker for Abbvie, Janssen‐Cilag, MSD, Takeda, Celltrion, Teva, Sandoz and Dr. Falk, received funding (Grants/Honoraria) from Dr. Falk, Janssen‐Cilag, Abbvie, Takeda.Consulting and fees from Celgene, all outside the submitted work. A.B. has served on the advisory board of Takeda, Abbvie and Janssen, outside the submitted work. G.D. reports grant support from DSM nutritional products LTD and speakers fees from Janssen Pharmaceuticals, Abbvie and Takeda, outside the submitted work. N.dB. has served as a speaker for AbbVie and M.S.D. and has served as consultant and principal investigator for TEVA Pharma BV and Takeda, and has received an (unrestricted) research grant from Dr. Falk, TEVA Pharma BV, MLDS and Takeda, all outside the submitted work. L.S. has served as a speaker and received research support from Takeda, outside the submitted work. A.vd.M. reports presentation fee from Janssen and has served on the advisory board of Takeda and Galapagos, outside the submitted work. R.W. has served on the advisory board and as invited speaker for Janssen, Pfizer and Takeda, outside the submitted work. C.vd.W. received grant support from Falk Benelux and Pfizer; received speaker fees from AbbVie, Takeda, Ferring, Dr. Falk Pharma, Hospira, Pfizer; and served as a consultant for AbbVie, MSD, Takeda, Celgene, Mundipharma and Janssen, outside the submitted work. O.v.R. has served as invited speaker for Janssen‐Cilag and has received nonfinancial support from Takeda, outside the submitted work. A.d.V. has served on advisory boards for Takeda, Janssen, Bristol Myers Squibb, Abbvie, Pfizer, and Galapagos and has received unrestricted research grants from Takeda, Janssen, and Pfizer, outside the submitted work. All other authors report no conflicts of interests.
Figures



References
-
- Gionchetti P., Dignass A., Danese S., et al., “3rd European Evidence‐Based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 2: Surgical Management and Special Situations,” Journal of Crohn's & Colitis 11 (2017): 135–149. - PubMed
-
- Nguyen G. C., E. V. Loftus, Jr. , Hirano I., et al., “American Gastroenterological Association Institute Guideline on the Management of Crohn's Disease After Surgical Resection,” Gastroenterology 152 (2017): 271–275. - PubMed
-
- López‐Sanromán A., Vera‐Mendoza I., Domènech E., et al., “Adalimumab vs Azathioprine in the Prevention of Postoperative Crohn's Disease Recurrence. A GETECCU Randomised Trial,” Journal of Crohn's & Colitis 11 (2017): 1293–1301. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
