Binding and cleavage of pro-urokinase by a tegument extract of Fasciola hepatica newly excysted juveniles activate the host fibrinolytic system
- PMID: 39856784
- PMCID: PMC11762853
- DOI: 10.1186/s13567-025-01449-4
Binding and cleavage of pro-urokinase by a tegument extract of Fasciola hepatica newly excysted juveniles activate the host fibrinolytic system
Abstract
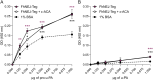
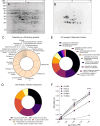
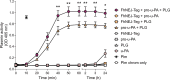
Plasmin, the final product of fibrinolysis, is a broad-spectrum serine protease that degrades extracellular matrix (ECM) components, a function exploited by multiple pathogens for dissemination purposes. The trematode Fasciola hepatica is the leading cause of fasciolosis, a major disease of livestock and an emerging zoonosis in humans. Infection success depends on the ability of F. hepatica newly excysted juveniles (FhNEJ) to penetrate the host intestinal wall, a process that remains incompletely understood. We have previously shown that FhNEJ are capable of binding plasminogen (PLG), the zymogen of plasmin, on their tegument surface, which leads to plasmin generation in the presence of host-derived PLG activators and subsequent degradation of laminin, a major component of the intestinal ECM. Here, we describe the interaction between a tegument extract of FhNEJ and the precursor of the urokinase-type PLG activator (pro-u-PA). We found that F. hepatica cathepsins B3, L3, enolase and glutathione S-transferase mediate this interaction, suggesting a multifactorial or moonlighting role for these proteins. Additionally, our results revealed that the tegument of FhNEJ contains a protease that is capable of cleaving and activating pro-u-PA into its catalytically active form, which positively impacts the capacity of the parasites to generate plasmin from the host PLG. Collectively, our findings indicate that FhNEJ interact with the host fibrinolytic system at multiple levels, reinforcing the potential of targeting this interaction as a strategy to prevent FhNEJ trans-intestinal migration and infection success.
Keywords: Fasciola hepatica; fibrinolytic system; host‒parasite interactions; newly excysted juveniles; pro-urokinase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- González-Miguel J, Siles-Lucas M, Kartashev V, Morchón R, Simón F (2016) Plasmin in parasitic chronic infections: friend or foe? Trends Parasitol 32:325–335 - PubMed
-
- Figuera L, Gómez-Arreaza A, Avilán L (2013) Parasitism in optima forma: exploiting the host fibrinolytic system for invasion. Acta Trop 128:116–123 - PubMed
-
- Cesarman-Maus G, Hajjar KA (2005) Molecular mechanisms of fibrinolysis. Br J Haematol 129:307–321 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

