Revealing the Mechanism of Protein Degradation in Postmortem Meat: The Role of Phosphorylation and Ubiquitination
- PMID: 39856851
- PMCID: PMC11764534
- DOI: 10.3390/foods14020184
Revealing the Mechanism of Protein Degradation in Postmortem Meat: The Role of Phosphorylation and Ubiquitination
Abstract
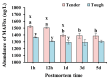
The aim of this study was to investigate the possible effects of phosphorylation and ubiquitination on the degradation of myofibrillar proteins in mutton with different tenderness. The longissimus thoracis lumborum muscles were chosen and divided into tender and tough groups (n = 9), and then stored at 4 °C for 1 h, 12 h, 1 d, 3 d, and 5 d postmortem. Shear force, pH, myofibril fragmentation index, AMPK activity, E3 ubiquitin ligase abundance, protein phosphorylation, and the ubiquitination levels of muscle samples were measured. The results demonstrated that the meat of samples in the tender group had a higher degradation of desmin and a lower phosphorylation level of desmin at 1 d compared with the tough group. The ubiquitination level of desmin, AMPK activity, and E3 ubiquitin ligase abundance in the tender group were noticeably higher than those in the tough group at 12 h. There was a negative correlation between the shear force and desmin degradation. The desmin degradation was negatively correlated with desmin phosphorylation and ubiquitination levels. The phosphorylation level of desmin was positively correlated with its ubiquitination. In summary, this study suggests that AMPK and E3 ubiquitin ligase concurrently play significant roles in regulating meat tenderness by regulating phosphorylation and ubiquitination in meat postmortem.
Keywords: AMPK; E3 ubiquitin ligase; meat tenderness; protein posttranslational modifications.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources

