Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk
- PMID: 39856987
- PMCID: PMC11765231
- DOI: 10.3390/foods14020320
Changes in Microbial Safety and Quality of High-Pressure Processed Camel Milk
Abstract
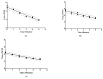
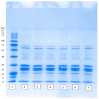
High-pressure processing (HPP) is used as a non-thermal approach for controlling microbial viability. The purposes of this study were to (i) establish the decimal reduction times (D-values) for pathogenic bacteria during 350 MPa HPP treatment,; (ii) evaluate the impact of 350 MPa HPP on total plate count (TPC), yeasts and molds (YM), and lactic acid bacteria (LAB) in camel milk; (iii) investigate the behavior of several spoilage-causing bacteria during storage at 4 °C and 10 °C for up to 10 d post-HPP treatment; and (iv) assess the effect of HPP on the protein degradation of camel milk. The D-values for L. monocytogenes, E. coli O157:H7, and Salmonella spp. were 3.77 ± 0.36 min, 1.48 ± 0.08 min, and 2.10 ± 0.13 min, respectively. The HPP treatment decreased pathogenic microorganisms by up to 2 to 3 log cfu/mL (depending on treatment conditions). However, HPP reduced TPC, YM, and LAB by <1 log cfu/mL, regardless of the length of pressure exposure. HPP treatment, even at extended holding times, did not significantly alter either the proteolytic activity or casein micelle structure in camel milk. This study highlights HPP as a promising non-thermal technique for enhancing the microbiological safety of camel milk.
Keywords: lactic acid bacteria; pathogens; proteins; spoilage causing microorganisms; total plate count; yeasts and molds.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Use of mild-heat treatment following high-pressure processing to prevent recovery of pressure-injured Listeria monocytogenes in milk.Food Microbiol. 2008 Apr;25(2):288-93. doi: 10.1016/j.fm.2007.10.009. Epub 2007 Oct 22. Food Microbiol. 2008. PMID: 18206771
-
Evaluation of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and Genoa salami.Int J Food Microbiol. 2010 May 30;140(1):61-75. doi: 10.1016/j.ijfoodmicro.2010.02.008. Epub 2010 Feb 13. Int J Food Microbiol. 2010. PMID: 20207436
-
Behavior of Escherichia coli O157:H7 and Listeria monocytogenes during fermentation and storage of camel yogurt.J Dairy Sci. 2016 Mar;99(3):1802-1811. doi: 10.3168/jds.2015-9872. Epub 2015 Dec 24. J Dairy Sci. 2016. PMID: 26723116
-
High-pressure processing as an emerging technology for enhancing dairy product safety and quality: a critical review of latest advances, sustainability impact, limitations, and prospects.Crit Rev Food Sci Nutr. 2025 May 6:1-25. doi: 10.1080/10408398.2025.2499623. Online ahead of print. Crit Rev Food Sci Nutr. 2025. PMID: 40326610 Review.
-
Impact of high-pressure processing on the bioactive compounds of milk - A comprehensive review.J Food Sci Technol. 2024 Sep;61(9):1632-1651. doi: 10.1007/s13197-024-05938-w. Epub 2024 Mar 6. J Food Sci Technol. 2024. PMID: 39049911 Free PMC article. Review.
References
-
- El-Aziz A., Kassem J.M., Aasem F.M., Abbas H.M. Physicochemical Properties and Health Benefits of Camel Milk and Its Applications in Dairy Products: A Review. Egypt. J. Chem. 2022;65:101–118. doi: 10.21608/ejchem.2021.92589.4383. - DOI
-
- Imarc UAE Camel Dairy Market Report by Product Type. [(accessed on 5 August 2024)]. Available online: https://www.imarcgroup.com/uae-camel-dairy-market.
-
- Seifu E. Camel Milk Products: Innovations, Limitations and Opportunities. Food Prod. Process. Nutr. 2023;5:15. doi: 10.1186/s43014-023-00130-7. - DOI
-
- Swelum A.A., El-Saadony M.T., Abdo M., Ombarak R.A., Hussein E.O.S., Suliman G., Alhimaidi A.R., Ammari A.A., Ba-Awadh H., Taha A.E. Nutritional, Antimicrobial and Medicinal Properties of Camel’s Milk: A Review. Saudi J. Biol. Sci. 2021;28:3126–3136. doi: 10.1016/j.sjbs.2021.02.057. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

