Comments and Illustrations of the European Federation of Societies for Ultrasound in Medicine Guidelines: Benign Pleura Lesions (Benign Pleura Thickening, Lesions and Masses)-What Can Be Seen on Transthoracic Ultrasound?
- PMID: 39857060
- PMCID: PMC11763749
- DOI: 10.3390/diagnostics15020176
Comments and Illustrations of the European Federation of Societies for Ultrasound in Medicine Guidelines: Benign Pleura Lesions (Benign Pleura Thickening, Lesions and Masses)-What Can Be Seen on Transthoracic Ultrasound?
Abstract
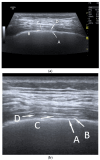
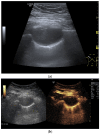
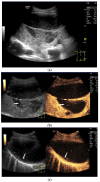
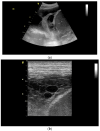
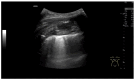
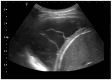
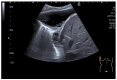
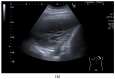
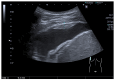
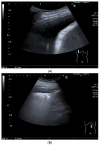
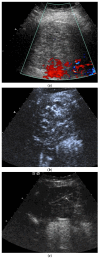
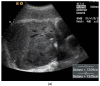
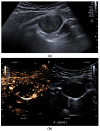
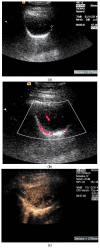
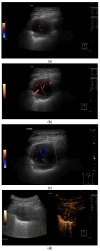
Pleural thickening can be the result of inflammation or infection but can also have a neoplastic origin. Depending on the clinical context, a pleural lesion or mass is often initially suspected of malignancy. Benign pleural tumors are rare, and their appearance on ultrasound (US) is also described less frequently than pleural metastases or malignancies. There are few descriptions of contrast-enhanced Ultrasound (CEUS) in particular. This review introduces the basics of transthoracic ultrasound (TUS) of the pleura and CEUS of the pleura and lung. CEUS is recommended for pulmonary applications in the EFSUMB guidelines in non-hepatic applications. This article provides an overview of the characteristics of benign pleural thickening, tumor-like lesions, and benign pleural tumors on transthoracic B-mode US with color Doppler imaging (CDI) and CEUS. In detail, characteristics in TUS and CEUS are described for infectious/inflammatory pleural thickening (empyema, tuberculous pleuritis, hemothorax, fibrothorax), pleural thickening in various systemic diseases, in tumor-like conditions (plaques, splenosis, endometriosis, mesothelial cysts, lymphangiomatosis) and benign tumors (lipoma, benign SFT, schwannoma, solitary extramedullary/extraosseous plasmacytoma). The descriptions are illustrated by corresponding US and CEUS images.
Keywords: benign pleural tumors; benign tumorlike conditions; contrast-enhanced ultrasonography; pleural lesions; transthoracic ultrasonography.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report. Some authors have received financial support and/or honoraria from Bracco for the organization of ultrasound courses. In addition, some authors have been supported with equipment from various ultrasound equipment companies for the organization of ultrasound courses and/or have received honoraria for lectures.
Figures






References
-
- Safai Zadeh E., Gorg C., Prosch H., Horn R., Jenssen C., Dietrich C.F. The Role of Thoracic Ultrasound for Diagnosis of Diseases of the Chest Wall, the Mediastinum, and the Diaphragm-Narrative Review and Pictorial Essay. Diagnostics. 2023;13:767. doi: 10.3390/diagnostics13040767. - DOI - PMC - PubMed
-
- Zadeh E.S., Dietrich C.F., Kmoth L., Trenker C., Alhyari A., Ludwig M., Görg C. Peripheral Pulmonary Lesions in Confirmed Pulmonary Arterial Embolism: Follow-up Study of B-Mode Ultrasound and of Perfusion Patterns Using Contrast-Enhanced Ultrasound (CEUS) J. Ultrasound Med. 2022;41:1713–1721. doi: 10.1002/jum.15852. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

