Machine-Learning Parsimonious Prediction Model for Diagnostic Screening of Severe Hematological Adverse Events in Cancer Patients Treated with PD-1/PD-L1 Inhibitors: Retrospective Observational Study by Using the Common Data Model
- PMID: 39857110
- PMCID: PMC11763827
- DOI: 10.3390/diagnostics15020226
Machine-Learning Parsimonious Prediction Model for Diagnostic Screening of Severe Hematological Adverse Events in Cancer Patients Treated with PD-1/PD-L1 Inhibitors: Retrospective Observational Study by Using the Common Data Model
Abstract
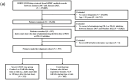
Background/Objectives: Earlier detection of severe immune-related hematological adverse events (irHAEs) in cancer patients treated with a PD-1 or PD-L1 inhibitor is critical to improving treatment outcomes. The study aimed to develop a simple machine learning (ML) model for predicting irHAEs associated with PD-1/PD-L1 inhibitors. Methods: We utilized the Observational Medical Outcomes Partnership-Common Data Model based on electronic medical records from a tertiary (KHMC) and a secondary (KHNMC) hospital in South Korea. Severe irHAEs were defined as Grades 3-5 by the Common Terminology Criteria for Adverse Events (version 5.0). The predictive model was developed using the KHMC dataset, and then cross-validated against an independent cohort (KHNMC). The full ML models were then simplified by selecting critical features based on the feature importance values (FIVs). Results: Overall, 397 and 255 patients were included in the primary (KHMC) and cross-validation (KHNMC) cohort, respectively. Among the tested ML algorithms, random forest achieved the highest accuracy (area under the receiver operating characteristic curve [AUROC] 0.88 for both cohorts). Parsimonious models reduced to 50% FIVs of the full models showed comparable performance to the full models (AUROC 0.83-0.86, p > 0.05). The KHMC and KHNMC parsimonious models shared common predictive features including furosemide, oxygen gas, piperacillin/tazobactam, and acetylcysteine. Conclusions: Considering the simplicity and adequate predictive performance, our simplified ML models might be easily implemented in clinical practice with broad applicability. Our model might enhance early diagnostic screening of irHAEs induced by PD-1/PD-L1 inhibitors, contributing to minimizing the risk of severe irHAEs and improving the effectiveness of cancer immunotherapy.
Keywords: PD-1 inhibitor; PD-L1 inhibitor; common data model (CDM); immune checkpoint inhibitor; immune-related hematological adverse events; machine learning; observational medical outcomes partnership (OMOP); parsimonious model; pharmacovigilance; real-world data (RWD); risk prediction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Hospital Length of Stay Prediction for Planned Admissions Using Observational Medical Outcomes Partnership Common Data Model: Retrospective Study.J Med Internet Res. 2024 Nov 22;26:e59260. doi: 10.2196/59260. J Med Internet Res. 2024. PMID: 39576284 Free PMC article.
-
A two-stage ensemble learning based prediction and grading model for PD-1/PD-L1 inhibitor-related cardiac adverse events: A multicenter retrospective study.Comput Methods Programs Biomed. 2024 Oct;255:108360. doi: 10.1016/j.cmpb.2024.108360. Epub 2024 Aug 5. Comput Methods Programs Biomed. 2024. PMID: 39163785
-
A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study.Lancet Oncol. 2018 Sep;19(9):1180-1191. doi: 10.1016/S1470-2045(18)30413-3. Epub 2018 Aug 14. Lancet Oncol. 2018. PMID: 30120041
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
References
-
- Wojtukiewicz M.Z., Rek M.M., Karpowicz K., Górska M., Polityńska B., Wojtukiewicz A.M., Moniuszko M., Radziwon P., Tucker S.C., Honn K.V. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—New opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40:949–982. doi: 10.1007/s10555-021-09976-0. - DOI - PMC - PubMed
-
- Ruggiero R., Fraenza F., Scavone C., di Mauro G., Piscitelli R., Mascolo A., Ferrajolo C., Rafaniello C., Sportiello L., Rossi F., et al. Immune Checkpoint Inhibitors and Immune-Related Adverse Drug Reactions: Data From Italian Pharma-covigilance Database. Front. Pharmacol. 2020;11:830. doi: 10.3389/fphar.2020.00830. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

