Nicotinamide Mononucleotide Restores NAD+ Levels to Alleviate LPS-Induced Inflammation via the TLR4/NF-κB/MAPK Signaling Pathway in Mice Granulosa Cells
- PMID: 39857373
- PMCID: PMC11762685
- DOI: 10.3390/antiox14010039
Nicotinamide Mononucleotide Restores NAD+ Levels to Alleviate LPS-Induced Inflammation via the TLR4/NF-κB/MAPK Signaling Pathway in Mice Granulosa Cells
Abstract
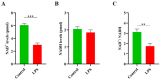
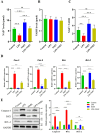
Inflammation disrupts the normal function of granulosa cells (GCs), which leads to ovarian dysfunction and fertility decline. Inflammatory conditions such as polycystic ovary syndrome (PCOS), primary ovarian insufficiency (POI), endometriosis, and age-related ovarian decline are often associated with chronic low-grade inflammation. Nicotinamide mononucleotide (NMN) is an important precursor of NAD+ and has gained attention for its potential to modulate cellular metabolism, redox homeostasis, and mitigate inflammation. This study investigated the protective roles of NMN against lipopolysaccharide LPS-mediated inflammation in GCs. The results of this experiment demonstrated that LPS had negative effects on GCs in term of reduced viability and proliferation rates and upregulated the production of pro-inflammatory cytokines, including interleukin-1 beta (IL-1β), interleukin-6 (IL-6), cyclooxygenase-2 (Cox-2), and tumor necrosis factor-alpha (TNF-α). Notably, the levels of NAD+ and NAD+/NADH ratio in GCs were reduced in response to inflammation. On the other hand, NMN supplementation restored the NAD+ levels and the NAD+/NADH ratio in GCs and significantly reduced the expression of pro-inflammatory markers at both mRNA and protein levels. It also enhanced cell viability and proliferation rates of GCs. Furthermore, NMN also reduced apoptosis rates in GCs by downregulating pro-apoptotic markers, including Caspase-3, Caspase-9, and Bax while upregulating anti-apoptotic marker Bcl-2. NMN supplementation significantly reduced reactive oxygen species ROS and improved steroidogenesis activity by restoring the estradiol (E2) and progesterone (P4) levels in LPS-treated GCs. Mechanistically, this study found that NMN suppressed the activation of the TLR4/NF-κB/MAPK signaling pathways in GCs, which regulates inflammatory processes. In conclusion, the findings of this study revealed that NMN has the potential to reduce LPS-mediated inflammatory changes in GCs by modulating NAD+ metabolism and inflammatory signaling pathways. NMN supplementation can be used as a potential therapeutic agent for ovarian inflammation and related fertility disorders.
Keywords: NAD+; NMN; ROS; apoptosis; granulosa cell; inflammation; steroidogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Cattelan A., Ceolotto G., Bova S., Albiero M., Kuppusamy M., De Martin S., Semplicini A., Fadini G.P., de Kreutzenberg S.V., Avogaro A. NAD+-dependent SIRT1 deactivation has a key role on ischemia–reperfusion-induced apoptosis. Vasc. Pharmacol. 2015;70:35–44. doi: 10.1016/j.vph.2015.02.004. - DOI - PubMed
-
- Zhao Y., Zhang J., Zheng Y., Zhang Y., Zhang X.J., Wang H., Du Y., Guan J., Wang X., Fu J. NAD+ improves cognitive function and reduces neuroinflammation by ameliorating mitochondrial damage and decreasing ROS production in chronic cerebral hypoperfusion models through Sirt1/PGC-1α pathway. J. Neuroinflamm. 2021;18:207. doi: 10.1186/s12974-021-02250-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

