Gut Microbiota at the Crossroad of Hepatic Oxidative Stress and MASLD
- PMID: 39857390
- PMCID: PMC11759774
- DOI: 10.3390/antiox14010056
Gut Microbiota at the Crossroad of Hepatic Oxidative Stress and MASLD
Abstract
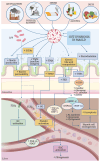
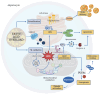
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a prevalent chronic liver condition marked by excessive lipid accumulation in hepatic tissue. This disorder can lead to a range of pathological outcomes, including metabolic dysfunction-associated steatohepatitis (MASH) and cirrhosis. Despite extensive research, the molecular mechanisms driving MASLD initiation and progression remain incompletely understood. Oxidative stress and lipid peroxidation are pivotal in the "multiple parallel hit model", contributing to hepatic cell death and tissue damage. Gut microbiota plays a substantial role in modulating hepatic oxidative stress through multiple pathways: impairing the intestinal barrier, which results in bacterial translocation and chronic hepatic inflammation; modifying bile acid structure, which impacts signaling cascades involved in lipidic metabolism; influencing hepatocytes' ferroptosis, a form of programmed cell death; regulating trimethylamine N-oxide (TMAO) metabolism; and activating platelet function, both recently identified as pathogenetic factors in MASH progression. Moreover, various exogenous factors impact gut microbiota and its involvement in MASLD-related oxidative stress, such as air pollution, physical activity, cigarette smoke, alcohol, and dietary patterns. This manuscript aims to provide a state-of-the-art overview focused on the intricate interplay between gut microbiota, lipid peroxidation, and MASLD pathogenesis, offering insights into potential strategies to prevent disease progression and its associated complications.
Keywords: farnesoid X receptor; ferroptosis; gut microbiota; intestinal permeability; lipid peroxidation; metabolic dysfunction-associated steatotic liver disease; oxidative stress; platelet activation; trimethylamine N-oxide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Le P., Payne J.Y., Zhang L., Deshpande A., Rothberg M.B., Alkhouri N., Herman W., Hernandez A.V., Schleicher M., Ye W., et al. Disease State Transition Probabilities Across the Spectrum of NAFLD: A Systematic Review and Meta-Analysis of Paired Biopsy or Imaging Studies. Clin. Gastroenterol. Hepatol. 2023;21:1154–1168. doi: 10.1016/j.cgh.2022.07.033. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

