Advances in Fluorescence Techniques for the Detection of Hydroxyl Radicals near DNA and Within Organelles and Membranes
- PMID: 39857413
- PMCID: PMC11762621
- DOI: 10.3390/antiox14010079
Advances in Fluorescence Techniques for the Detection of Hydroxyl Radicals near DNA and Within Organelles and Membranes
Abstract
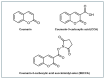
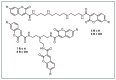
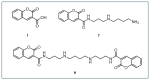
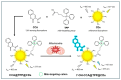
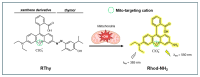
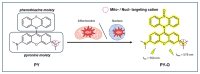
Hydroxyl radicals (•OH), the most potent oxidants among reactive oxygen species (ROS), are a major contributor to oxidative damage of biomacromolecules, including DNA, lipids, and proteins. The overproduction of •OH is implicated in the pathogenesis of numerous diseases such as cancer, neurodegenerative disorders, and some cardiovascular pathologies. Given the localized nature of •OH-induced damage, detecting •OH, specifically near DNA and within organelles, is crucial for understanding their pathological roles. The major challenge of •OH detection results from their short half-life, high reactivity, and low concentrations within biological systems. As a result, there is a growing need for the development of highly sensitive and selective probes that can detect •OH in specific cellular regions. This review focuses on the advances in fluorescence probes designed to detect •OH near DNA and within cellular organelles and membranes. The key designs of the probes are highlighted, with emphasis on their strengths, applications, and limitations. Recommendations for future research directions are given to further enhance probe development and characterization.
Keywords: DNA-targeting; coumarin-based probes; fluorescence detection; hydroxyl radicals; organelle-targeting; oxidative stress; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Use and Evaluation of Newly Synthesized Fluorescence Probes to Detect Generated OH• Radicals in Fibroblast Cells.J Fluoresc. 2016 May;26(3):919-24. doi: 10.1007/s10895-016-1780-9. Epub 2016 Mar 16. J Fluoresc. 2016. PMID: 26983614
-
An innovative dual-organelle targeting NIR fluorescence probe for detecting hydroxyl radicals in biosystem and inflammation models.Bioorg Chem. 2024 Oct;151:107678. doi: 10.1016/j.bioorg.2024.107678. Epub 2024 Jul 26. Bioorg Chem. 2024. PMID: 39068715
-
Dual-Activated Photoacoustic Probe for Reliably Detecting Hydroxyl Radical in Ischemic Cardiovascular Disease in Mouse and Human Samples.ACS Sens. 2024 Oct 25;9(10):5445-5453. doi: 10.1021/acssensors.4c01665. Epub 2024 Oct 4. ACS Sens. 2024. PMID: 39364916
-
Recent advances in electrochemical detection of reactive oxygen species: a review.Analyst. 2025 Apr 8;150(8):1490-1517. doi: 10.1039/d4an01533a. Analyst. 2025. PMID: 40151998 Review.
-
Recent advances in fluorescent probes for the detection of reactive oxygen species.Anal Bioanal Chem. 2006 Oct;386(3):532-43. doi: 10.1007/s00216-006-0366-9. Epub 2006 May 13. Anal Bioanal Chem. 2006. PMID: 16609844 Review.
References
-
- Lambert A.J., Brand M.D. Reactive oxygen species production by mitochondria. In: Stuart J.A., editor. Mitochondrial DNA. 2nd ed. Volume 554. Humana Press; Totowa, NJ, USA: 2009. pp. 165–181. Methods in Molecular BiologyTM. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

