Treating Metabolic Dysregulation and Senescence by Caloric Restriction: Killing Two Birds with One Stone?
- PMID: 39857433
- PMCID: PMC11763027
- DOI: 10.3390/antiox14010099
Treating Metabolic Dysregulation and Senescence by Caloric Restriction: Killing Two Birds with One Stone?
Abstract
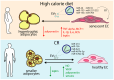
Cellular senescence is a state of permanent cell cycle arrest accompanied by metabolic activity and characteristic phenotypic changes. This process is crucial for developing age-related diseases, where excessive calorie intake accelerates metabolic dysfunction and aging. Overnutrition disturbs key metabolic pathways, including insulin/insulin-like growth factor signaling (IIS), the mammalian target of rapamycin (mTOR), and AMP-activated protein kinase. The dysregulation of these pathways contributes to insulin resistance, impaired autophagy, exacerbated oxidative stress, and mitochondrial dysfunction, further enhancing cellular senescence and systemic metabolic derangements. On the other hand, dysfunctional endothelial cells and adipocytes contribute to systemic inflammation, reduced nitric oxide production, and altered lipid metabolism. Numerous factors, including extracellular vesicles, mediate pathological communication between the vascular system and adipose tissue, amplifying metabolic imbalances. Meanwhile, caloric restriction (CR) emerges as a potent intervention to counteract overnutrition effects, improve mitochondrial function, reduce oxidative stress, and restore metabolic balance. CR modulates pathways such as IIS, mTOR, and sirtuins, enhancing glucose and lipid metabolism, reducing inflammation, and promoting autophagy. CR can extend the health span and mitigate age-related diseases by delaying cellular senescence and improving healthy endothelial-adipocyte interactions. This review highlights the crosstalk between endothelial cells and adipocytes, emphasizing CR potential in counteracting overnutrition-induced senescence and restoring vascular homeostasis.
Keywords: adipocytes; caloric restriction; endothelial dysfunction; oxidative stress; senescence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Beyene H.B., Giles C., Huynh K., Wang T., Cinel M., Mellett N.A., Olshansky G., Meikle T.G., Watts G.F., Hung J., et al. Metabolic phenotyping of BMI to characterize cardiometabolic risk: Evidence from large population-based cohorts. Nat. Commun. 2023;14:6280. doi: 10.1038/s41467-023-41963-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

