mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin
- PMID: 39857585
- PMCID: PMC11759183
- DOI: 10.3390/biomedicines13010001
mTOR Inhibitor Everolimus Modulates Tumor Growth in Small-Cell Carcinoma of the Ovary, Hypercalcemic Type and Augments the Drug Sensitivity of Cancer Cells to Cisplatin
Abstract
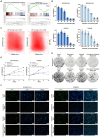
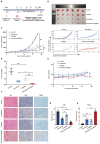
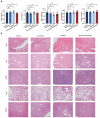
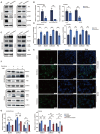
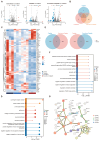
Background: Small-cell carcinoma of the ovary, hypercalcemic type (SCCOHT), is a rare and aggressive cancer with a poor prognosis and limited treatment options. Current chemotherapy regimens are predominantly platinum-based; however, the development of platinum resistance during treatment significantly worsens patient outcomes. Everolimus, an mTOR inhibitor, has been widely used in combination cancer therapies and has successfully enhanced the efficacy of platinum-based treatments. Method: In this study, we investigated the combined effects of everolimus and cisplatin on SCCOHT through both in vitro and in vivo experiments, complemented by RNA sequencing (RNA-seq) analyses to further elucidate the therapeutic impact. Result: Our findings revealed that everolimus significantly inhibits the proliferation of SCCOHT cells, induces cell cycle arrest, and accelerates apoptosis. When combined with cisplatin, everolimus notably enhances the therapeutic efficacy without increasing the toxicity typically associated with platinum-based drugs. RNA-seq analysis uncovered alterations in the expression of apoptosis-related genes, suggesting that the underlying mechanism involves autophagy regulation. Conclusions: Despite the current challenges in treating SCCOHT and the suboptimal efficacy of platinum-based therapies, the addition of everolimus significantly suppresses tumor growth. This indicates that everolimus enhances cisplatin efficacy by disrupting survival-promoting signaling cascades and inducing cell cycle arrest. Furthermore, it points to potential biomarkers for predicting therapeutic response.
Keywords: apoptosis; autophagy; combination therapy; hypercalcemic type; proliferation; small-cell carcinoma of the ovary; targeted therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Tischkowitz M., Huang S., Banerjee S., Hague J., Hendricks W.P.D., Huntsman D.G., Lang J.D., Orlando K.A., Oza A.M., Pautier P., et al. Small-Cell Carcinoma of the Ovary, Hypercalcemic Type-Genetics, New Treatment Targets, and Current Management Guidelines. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020;26:3908–3917. doi: 10.1158/1078-0432.CCR-19-3797. - DOI - PMC - PubMed
-
- Jamy O., Yaghmour G., Hare F., Martin M.G. Population-based Analysis of the Clinical Features of Primary Small Cell Carcinoma of the Ovary. Anticancer Res. 2015;35:3091–3095. - PubMed
-
- Harrison M.L., Hoskins P., du Bois A., Quinn M., Rustin G.J., Ledermann J.A., Baron-Hay S., Friedlander M.L. Small cell of the ovary, hypercalcemic type—Analysis of combined experience and recommendation for management. A GCIG study. Gynecol. Oncol. 2006;100:233–238. doi: 10.1016/j.ygyno.2005.10.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

