Extracellular Cold-Inducible RNA-Binding Protein and Hemorrhagic Shock: Mechanisms and Therapeutics
- PMID: 39857596
- PMCID: PMC11759867
- DOI: 10.3390/biomedicines13010012
Extracellular Cold-Inducible RNA-Binding Protein and Hemorrhagic Shock: Mechanisms and Therapeutics
Abstract
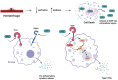
Hemorrhagic shock is a type of hypovolemic shock and a significant cause of trauma-related death worldwide. The innate immune system has been implicated as a key mediator in developing severe complications after shock. Inflammation from the innate immune system begins at the time of initial insult; however, its activation is exaggerated, resulting in early and late-stage complications. Hypoxia and hypoperfusion lead to the release of molecules that act as danger signals known as damage-associated molecular patterns (DAMPs). DAMPs continue to circulate after shock, resulting in excess inflammation and tissue damage. We recently discovered that cold-inducible RNA-binding protein released into the extracellular space acts as a DAMP. During hemorrhagic shock, hypoperfusion leads to cell necrosis and the release of CIRP into circulation, triggering both systemic inflammation and local tissue damage. In this review, we discuss extracellular cold-inducible RNA-binding protein (eCIRP)'s role in sterile inflammation, as well as its various mechanisms of action. We also share our more newly developed anti-eCIRP agents with the eventual goal of producing drug therapies to mitigate organ damage, reduce mortality, and improve patient outcomes related to hemorrhagic shock. Finally, we suggest that future preclinical studies are required to develop the listed therapeutics for hemorrhagic shock and related conditions. In addition, we emphasize on the challenges to the translational phase and caution that the therapy should allow the immune system to continue to function well against secondary infections during hospitalization.
Keywords: extracellular CIRP; inflammation; ischemia and reperfusion; organ damage; trauma hemorrhage.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Extracellular CIRP (eCIRP) and inflammation.J Leukoc Biol. 2019 Jul;106(1):133-146. doi: 10.1002/JLB.3MIR1118-443R. Epub 2019 Jan 15. J Leukoc Biol. 2019. PMID: 30645013 Free PMC article. Review.
-
Anti-DAMP therapies for acute inflammation.Front Immunol. 2025 May 8;16:1579954. doi: 10.3389/fimmu.2025.1579954. eCollection 2025. Front Immunol. 2025. PMID: 40406124 Free PMC article. Review.
-
Extracellular Cold-Inducible RNA-Binding Protein: Progress from Discovery to Present.Int J Mol Sci. 2025 Apr 9;26(8):3524. doi: 10.3390/ijms26083524. Int J Mol Sci. 2025. PMID: 40332009 Free PMC article. Review.
-
Extracellular CIRP induces acute kidney injury via endothelial TREM-1.Front Physiol. 2022 Sep 29;13:954815. doi: 10.3389/fphys.2022.954815. eCollection 2022. Front Physiol. 2022. PMID: 36246143 Free PMC article.
-
Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis.Nat Med. 2013 Nov;19(11):1489-1495. doi: 10.1038/nm.3368. Epub 2013 Oct 6. Nat Med. 2013. PMID: 24097189 Free PMC article.
Cited by
-
Rewiring of the glymphatic landscape in metabolic disorders.Trends Endocrinol Metab. 2025 Aug;36(8):710-720. doi: 10.1016/j.tem.2024.11.005. Epub 2024 Dec 4. Trends Endocrinol Metab. 2025. PMID: 39638721 Review.
-
Overview of methods that determine mitochondrial function in human disease.Metabolism. 2025 Sep;170:156300. doi: 10.1016/j.metabol.2025.156300. Epub 2025 May 17. Metabolism. 2025. PMID: 40389059 Free PMC article. Review.
-
Exosomal Proteomics: Unveiling Novel Insights into Lung Cancer.Aging Dis. 2024 Apr 9;16(2):876-900. doi: 10.14336/AD.2024.0409. Aging Dis. 2024. PMID: 38607736 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

