Outcomes of Idecabtagene Vicleucel Therapy in Patients with Relapsed/Refractory Multiple Myeloma: A Single-Institution Experience
- PMID: 39857619
- PMCID: PMC11759176
- DOI: 10.3390/biomedicines13010036
Outcomes of Idecabtagene Vicleucel Therapy in Patients with Relapsed/Refractory Multiple Myeloma: A Single-Institution Experience
Abstract
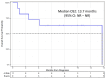
Background/Objectives: Idecabtagene vicleucel (ide-cel), an anti-B-cell maturation chimeric antigen receptor T-cell therapy, represents an unprecedented treatment option for relapsed/refractory multiple myeloma (R/R MM). Nevertheless, given its limitations, including the risk of adverse effects and unclear durability of efficacy, there remains a need to report the real-world clinical outcomes of ide-cel therapy in patients with R/R MM, as well as explore host predictive factors for therapy. Methods: We performed a single-center retrospective analysis of 25 adult patients with R/R MM who received ide-cel between 2021 and 2023 at the University of California San Diego Health. Data on baseline characteristics, efficacy, safety, and post-relapse outcomes were collected. Treatment responses were assessed using the International Myeloma Working Group criteria while survival analyses were conducted using the Kaplan-Meier and Cox proportional hazards methods. Results: The median age was 65. Twelve patients (48%) were male. Patients received a median of six lines of prior therapy with four patients (16%) receiving prior BCMA-targeted therapy. Six patients (24%) had high-risk cytogenetics while ten patients (40%) had extramedullary disease. The incidence of cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome incidence was 92% and 12%, respectively. All grade infection occurred in 11 patients (44%). Cytomegalovirus (CMV) reactivation occurred in 9 of 19 patients (47%) who were CMV IgG positive prior to CAR T-cell therapy. The objective response rate (ORR) was 84%; stringent complete response was seen in 14 patients (56%). After a median follow-up of 13 months, median progression-free survival (PFS) was 13.9 months (95% CI: 9.21 months-not reached [NR]); median overall survival (OS) was not reached (95% CI: 19.5 months-NR). Among the 11 patients (44%) who progressed after ide-cel therapy, median OS2 was 13.7 months; especially poor outcomes (median OS2 of 1.74 months) were observed in four patients who did not respond to ide-cel. Six of these eleven patients remained alive at time of data cutoff. Univariate and multivariate analysis revealed no significant predictors of ORR, PFS, or OS. Conclusions: Overall, ide-cel had comparable efficacy and safety to the KarMMa-1 trial and other reported real-world experiences.
Keywords: CAR T-cell therapy; CRS; ICANS; cytomegalovirus; ide-cel; multiple myeloma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials

