Angiotensin IV Receptors in the Rat Prefrontal Cortex: Neuronal Expression and NMDA Inhibition
- PMID: 39857655
- PMCID: PMC11760436
- DOI: 10.3390/biomedicines13010071
Angiotensin IV Receptors in the Rat Prefrontal Cortex: Neuronal Expression and NMDA Inhibition
Abstract
Background: N-methyl-D-aspartate type glutamate receptors (NMDARs) are fundamental to neuronal physiology and pathophysiology. The prefrontal cortex (PFC), a key region for cognitive function, is heavily implicated in neuropsychiatric disorders, positioning the modulation of its glutamatergic neurotransmission as a promising therapeutic target. Our recently published findings indicate that AT1 receptor activation enhances NMDAR activity in layer V pyramidal neurons of the rat PFC. At the same time, it suggests that alternative angiotensin pathways, presumably involving AT4 receptors (AT4Rs), might exert inhibitory effects. Angiotensin IV (Ang IV) and its analogs have demonstrated cognitive benefits in animal models of learning and memory deficits.
Methods: Immunohistochemistry and whole-cell patch-clamp techniques were used to map the cell-type-specific localization of AT4R, identical to insulin-regulated aminopeptidase (IRAP), and to investigate the modulatory effects of Ang IV on NMDAR function in layer V pyramidal cells of the rat PFC.
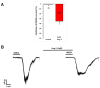
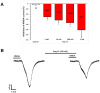
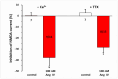
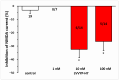
Results: AT4R/IRAP expression was detected in pyramidal cells and GABAergic interneurons, but not in microglia or astrocytes, in layer V of the PFC in 9-12-day-old and 6-month-old rats. NMDA (30 μM) induced stable inward cation currents, significantly inhibited by Ang IV (1 nM-1 µM) in a subset of pyramidal neurons. This inhibition was reproduced by the IRAP inhibitor LVVYP-H7 (10-100 nM). Synaptic isolation of pyramidal neurons did not affect the Ang IV-mediated inhibition of NMDA currents.
Conclusions: Ang IV/IRAP-mediated inhibition of NMDA currents in layer V pyramidal neurons of the PFC may represent a way of regulating cognitive functions and thus a potential pharmacological target for cognitive impairments and related neuropsychiatric disorders.
Keywords: AT4 angiotensin receptor; N-methyl-D-aspartate receptor; insulin-regulated aminopeptidase; neuromodulation; prefrontal cortex; renin–angiotensin system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Potentiation of NMDA Receptors by AT1 Angiotensin Receptor Activation in Layer V Pyramidal Neurons of the Rat Prefrontal Cortex.Int J Mol Sci. 2024 Nov 25;25(23):12644. doi: 10.3390/ijms252312644. Int J Mol Sci. 2024. PMID: 39684355 Free PMC article.
-
NR2A-Containing NMDARs in the Prefrontal Cortex Are Required for Working Memory and Associated with Age-Related Cognitive Decline.J Neurosci. 2016 Dec 14;36(50):12537-12548. doi: 10.1523/JNEUROSCI.2332-16.2016. Epub 2016 Nov 2. J Neurosci. 2016. PMID: 27807032 Free PMC article.
-
Involvement of the AT1 receptor subtype in the effects of angiotensin IV and LVV-haemorphin 7 on hippocampal neurotransmitter levels and spatial working memory.J Neurochem. 2010 Mar;112(5):1223-34. doi: 10.1111/j.1471-4159.2009.06547.x. Epub 2009 Dec 17. J Neurochem. 2010. PMID: 20028450
-
Integration of neuronal and glial signalling by pyramidal cells of the rat prefrontal cortex; control of cognitive functions and addictive behaviour by purinergic mechanisms.Neuropsychopharmacol Hung. 2013 Dec;15(4):206-13. Neuropsychopharmacol Hung. 2013. PMID: 24380961 Review.
-
Involvement of insulin-regulated aminopeptidase in the effects of the renin-angiotensin fragment angiotensin IV: a review.Heart Fail Rev. 2008 Sep;13(3):321-37. doi: 10.1007/s10741-007-9062-x. Epub 2007 Nov 8. Heart Fail Rev. 2008. PMID: 17990104 Review.
Cited by
-
Angiotensin II and Cardiovascular Disease: Balancing Pathogenic and Protective Pathways.Curr Issues Mol Biol. 2025 Jul 1;47(7):501. doi: 10.3390/cimb47070501. Curr Issues Mol Biol. 2025. PMID: 40728970 Free PMC article. Review.
-
GPCR Sense Communication Among Interaction Nematodes with Other Organisms.Int J Mol Sci. 2025 Mar 20;26(6):2822. doi: 10.3390/ijms26062822. Int J Mol Sci. 2025. PMID: 40141464 Free PMC article. Review.
References
-
- Fuster J.M. In: Chapter 1—Introduction, in The Prefrontal Cortex. 4th ed. Fuster J.M., editor. Academic Press; San Diego, CA, USA: 2008. pp. 1–6.
-
- Fuster J.M. In: Chapter 2—Anatomy of the Prefrontal Cortex, in The Prefrontal Cortex. 4th ed. Fuster J.M., editor. Academic Press; San Diego, CA, USA: 2008. pp. 7–58.
Grants and funding
- TKP2021-EGA-23/National Research, Development, and Innovation Fund (Hungary)
- TKP2021-EGA-25/National Research, Development, and Innovation Fund (Hungary)
- OTKA-K128875/National Research, Development, and Innovation Office of Hungary
- NKFI K 146086/National Research, Development, and Innovation Office of Hungary
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

