Modeling of Blood Flow Dynamics in Rat Somatosensory Cortex
- PMID: 39857656
- PMCID: PMC11761867
- DOI: 10.3390/biomedicines13010072
Modeling of Blood Flow Dynamics in Rat Somatosensory Cortex
Abstract
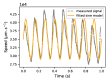
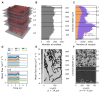
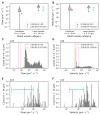
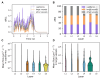
Background: The cerebral microvasculature forms a dense network of interconnected blood vessels where flow is modulated partly by astrocytes. Increased neuronal activity stimulates astrocytes to release vasoactive substances at the endfeet, altering the diameters of connected vessels. Methods: Our study simulated the coupling between blood flow variations and vessel diameter changes driven by astrocytic activity in the rat somatosensory cortex. We developed a framework with three key components: coupling between the vasculature and synthesized astrocytic morphologies, a fluid dynamics model to compute flow in each vascular segment, and a stochastic process replicating the effect of astrocytic endfeet on vessel radii. Results: The model was validated against experimental flow values from the literature across cortical depths. We found that local vasodilation from astrocyte activity increased blood flow, especially in capillaries, exhibiting a layer-specific response in deeper cortical layers. Additionally, the highest blood flow variability occurred in capillaries, emphasizing their role in cerebral perfusion regulation. We discovered that astrocytic activity impacted blood flow dynamics in a localized, clustered manner, with most vascular segments influenced by two to three neighboring endfeet. Conclusions: These insights enhance our understanding of neurovascular coupling and guide future research on blood flow-related diseases.
Keywords: astrocytic endfoot; blood flow; neuro-glia-vasculature; simulation; vasodilation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Kacem K., Lacombe P., Seylaz J., Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia. 1998;23:1–10. doi: 10.1002/(SICI)1098-1136(199805)23:1<1::AID-GLIA1>3.0.CO;2-B. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

