Therapeutic Potential of Targeting Ferroptosis in Periprosthetic Osteolysis Induced by Ultra-High-Molecular-Weight Polyethylene Wear Debris
- PMID: 39857757
- PMCID: PMC11762349
- DOI: 10.3390/biomedicines13010170
Therapeutic Potential of Targeting Ferroptosis in Periprosthetic Osteolysis Induced by Ultra-High-Molecular-Weight Polyethylene Wear Debris
Abstract
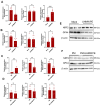
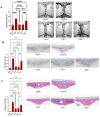
Background/Objectives: Periprosthetic osteolysis is the primary cause of arthroplasty failure in the majority of patients. Mechanistically, wear debris released from the articulating surfaces of a prosthesis initiates local inflammation and several modes of regulated cell death programs, such as ferroptosis, which represents a promising therapeutic target in various chronic inflammatory diseases. Thus, the current study aimed at exploring the therapeutic potential of targeting ferroptosis in a polyethylene-wear-debris-induced osteolysis model. Methods: Inverted cell culture model was used for stimulating the cells with wear debris in vitro, and calvarial osteolysis model was used for evaluating the therapeutic effects of inhibitors in vivo. Results: The immunostaining of periprosthetic bone tissues demonstrated a number of osteocytes expressing ferroptosis markers. Likewise, the expressions of ferroptosis markers were confirmed in polyethylene-wear-debris-stimulated osteocyte-like cells and primary osteoblasts in a direct stimulation model but not in an indirect stimulation model. Furthermore, polyethylene wear debris was implanted onto calvarial bone and mice were treated with the ferroptosis inhibitors DFO and Fer-1. These treatments alleviated the inflammatory and pathological bone resorption induced by the wear debris implantation. Conclusions: Our data broaden the knowledge of the pathogenesis of periprosthetic osteolysis and highlight ferroptosis as a promising therapeutic target.
Keywords: ferroptosis inhibitors; periprosthetic osteolysis; therapeutics; wear debris.
Conflict of interest statement
Author Tomoyo Yutani was employed by the Teijin Nakashima Medical Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Blockade of XCL1/Lymphotactin Ameliorates Severity of Periprosthetic Osteolysis Triggered by Polyethylene-Particles.Front Immunol. 2020 Aug 4;11:1720. doi: 10.3389/fimmu.2020.01720. eCollection 2020. Front Immunol. 2020. PMID: 32849609 Free PMC article.
-
Evidence that osteocyte perilacunar remodelling contributes to polyethylene wear particle induced osteolysis.Acta Biomater. 2016 Mar;33:242-51. doi: 10.1016/j.actbio.2016.01.016. Epub 2016 Jan 18. Acta Biomater. 2016. PMID: 26796208
-
Osteocytes respond to particles of clinically-relevant conventional and cross-linked polyethylene and metal alloys by up-regulation of resorptive and inflammatory pathways.Acta Biomater. 2019 Mar 15;87:296-306. doi: 10.1016/j.actbio.2019.01.047. Epub 2019 Jan 25. Acta Biomater. 2019. PMID: 30690207
-
Immunobiology of periprosthetic inflammation and pain following ultra-high-molecular-weight-polyethylene wear debris in the lumbar spine.Expert Rev Clin Immunol. 2018 Aug;14(8):695-706. doi: 10.1080/1744666X.2018.1511428. Epub 2018 Aug 21. Expert Rev Clin Immunol. 2018. PMID: 30099915 Free PMC article. Review.
-
Current research in the pathogenesis of aseptic implant loosening associated with particulate wear debris.Acta Orthop Belg. 2013 Feb;79(1):1-9. Acta Orthop Belg. 2013. PMID: 23547507 Review.
References
-
- Terkawi M.A., Matsumae G., Shimizu T., Takahashi D., Kadoya K., Iwasaki N. Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives. Int. J. Mol. Sci. 2022;23:1786. doi: 10.3390/ijms23031786. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

