SSTR2-Targeted Theranostics in Hepatocellular Carcinoma
- PMID: 39857944
- PMCID: PMC11763341
- DOI: 10.3390/cancers17020162
SSTR2-Targeted Theranostics in Hepatocellular Carcinoma
Abstract
Background: While the clinical use of radiolabeled somatostatin analogs is well established in neuroendocrine tumors, there is growing interest in expanding their application to other somatostatin receptor 2 (SSTR2)-expressing cancers. This study investigates the potential utility of SSTR2-targeted theranostics in hepatocellular carcinoma (HCC).
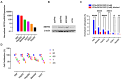
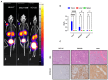
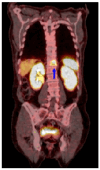
Methods: SSTR2 expression in HCC cell lines and clinical samples was evaluated using qRT-PCR, Western blot analysis, and a public dataset. 67Ga-DOTATATE uptake was measured, 177Lu-DOTATATE cytotoxicity was assessed, and 68Ga-DOTATATE tumor targeting was evaluated in HCC animal models and a patient via PET/CT imaging.
Results: SSTR2 expression was confirmed in HCC cell lines and clinical samples. Radioligand uptake studies demonstrated SSTR2-mediated 67Ga-DOTATATE uptake. 177Lu-DOTATATE treatment reduced cell proliferation and enhanced the anti-tumor efficacy of the multikinase inhibitor sorafenib. 68Ga-DOTATATE PET/CT scans successfully identified tumors in HCC animal models and spinal metastases in a patient with HCC.
Conclusion: These findings provide evidence that SSTR2-based theranostics could have significant implications for the detection and treatment of HCC.
Keywords: SSTR2; hepatocellular carcinoma; theranostics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Ginj M., Zhang H., Waser B., Cescato R., Wild D., Wang X., Erchegyi J., Rivier J., Macke H.R., Reubi J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA. 2006;103:16436–16441. doi: 10.1073/pnas.0607761103. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

