An Updated Systematic Review and Network Meta-Analysis of First-Line Triplet vs. Doublet Therapies for Metastatic Hormone-Sensitive Prostate Cancer
- PMID: 39857987
- PMCID: PMC11763793
- DOI: 10.3390/cancers17020205
An Updated Systematic Review and Network Meta-Analysis of First-Line Triplet vs. Doublet Therapies for Metastatic Hormone-Sensitive Prostate Cancer
Abstract
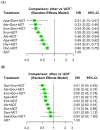
Purpose: The addition of androgen receptor pathway inhibitors (ARPIs) to androgen deprivation therapy (ADT), with or without docetaxel (Doc), is currently recommended for metastatic, hormone-sensitive prostate cancer (mHSPC). Recently, the ARANOTE trial evaluated the efficacy and safety of Darolutamide + ADT in this setting. We aimed to update a network meta-analysis (NMA) of these combination therapies. Methods: We conducted a systematic search for RCTs on systemic therapies for mHSPC using MEDLINE, Embase, and the Web of Science Core Collection in September 2024. An NMA utilizing random-effects models was performed to compare progression-free survival (PFS), overall survival (OS), and adverse event (AE) incidence (PROSPERO: CRD42024591458). Results: A total of 12 RCTs (n = 11,954) were included in our NMAs. Triplet therapies were associated with significant improvements in PFS compared to ARPI-based doublet therapies (hazard ratio [HR]: 0.74; 95% confidence interval [CI]: 0.59-0.93; p = 0.01), but the difference did not reach the conventional levels of statistical significance for OS (HR: 0.82; 95% CI: 0.67-1.01; p = 0.059). In a subset analysis, compared to ARPI-based doublet therapies, triplet therapies showed a significant improvement in PFS in patients with high-volume disease (HR: 0.64; 95% CI: 0.47-0.88; p < 0.01), whereas no significant improvement was observed in those with low-volume disease (HR: 0.86; 95% CI: 0.45-1.67; p = 0.7). No significant difference in grade ≥ 3 AEs was observed between triplet therapies and ARPI-based doublet therapies. The main limitations include patient heterogeneity and limited follow-up in some studies. Conclusions: Triplet therapies can improve the oncologic outcomes of patients with mHSPC compared to ARPI-based doublet therapies, without significantly increasing severe AEs. These findings warrant further confirmation in a head-to-head trial powered for overall survival.
Keywords: ARPI; adverse event; docetaxel; mHSPC; network meta-analysis; overall survival; progression-free survival; systematic review; triplet therapy.
Conflict of interest statement
Takahiro Kimura is a paid consultant/advisor of Astellas, Bayer, Janssen, and Sanofi. Shahrokh F. Shariat received the following: Honoraria: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Roche, and Takeda Consulting. Advisory Role: Astellas, AstraZeneca, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Pierre Fabre, Roche, and Takeda. Speakers Bureau: Astellas, Astra Zeneca, Bayer, BMS, Ferring, Ipsen, Janssen, MSD, Olympus, Pfizer, Richard Wolf, Roche, and Takeda. Pawel Rajwa is a paid consultant/advisor of Janssen. The other authors declare no conflicts of interest associated with this manuscript.
Figures



References
-
- Tilki D., van den Bergh R.C.N., Briers E., Van den Broeck T., Brunckhorst O., Darraugh J., Eberli D., De Meerleer G., De Santis M., Farolfi A., et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer. Part II-2024 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2024;86:164–182. doi: 10.1016/j.eururo.2024.04.010. - DOI - PubMed
-
- Matsukawa A., Rajwa P., Kawada T., Bekku K., Laukhtina E., Klemm J., Pradere B., Mori K., Karakiewicz P.I., Kimura T., et al. Impact of disease volume on survival efficacy of triplet therapy for metastatic hormone-sensitive prostate cancer: A systematic review, meta-analysis, and network meta-analysis. Int. J. Clin. Oncol. 2024;29:716–725. doi: 10.1007/s10147-024-02485-4. - DOI - PMC - PubMed
-
- Yanagisawa T., Rajwa P., Thibault C., Gandaglia G., Mori K., Kawada T., Fukuokaya W., Shim S.R., Mostafaei H., Motlagh R.S., et al. Androgen Receptor Signaling Inhibitors in Addition to Docetaxel with Androgen Deprivation Therapy for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2022;82:584–598. doi: 10.1016/j.eururo.2022.08.002. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

