A Multidisciplinary Approach to Diagnosing Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): Practical Recommendations and Insights from Countries of the Gulf Cooperation Council
- PMID: 39858003
- PMCID: PMC11763774
- DOI: 10.3390/cancers17020221
A Multidisciplinary Approach to Diagnosing Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN): Practical Recommendations and Insights from Countries of the Gulf Cooperation Council
Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is an aggressive orphan hematopoietic malignancy characterized by cutaneous and systemic hematologic involvement. BPDCN is frequently misidentified, but early, accurate diagnosis is critical to extending patient survival using tagraxofusp, a first-in-class CD123-targeted therapy, and increasing their chances of receiving a potentially curative stem cell transplantation. Cases of BPDCN in countries of the Gulf Cooperation Council are lower than the extrapolated incidence from other geographic locations due to lack of awareness of key diagnostic features, lack of consensus on the minimal phenotype for diagnosis, and lack of local immunohistochemistry testing facilities, contributing to underdiagnosis in this region. Practical recommendations, a streamlined diagnostic panel, and suggested multidisciplinary approaches based on expert experience regarding diagnostic and clinical challenges specific to this region, and a review of the literature are presented here to facilitate diagnosis of BPDCN in this region by primary care physicians, dermatologists, and hematologists.
Keywords: Gulf Cooperation Council; blastic plasmacytoid dendritic cell neoplasm; consensus recommendations; dendritic cells/pathology; differential diagnosis; hematologic neoplasms/diagnosis; tagraxofusp.
Conflict of interest statement
N.B. has received consulting fees from Stemline Therapeutics and travel expenses from BD Biosciences. R.P. has received honoraria from Janssen, Amgen, Novartis, Sanofi, AstraZeneca, and Takeda. She has also received consulting fees from Janssen, Servier, Amgen, Sobi, Novartis, and Pfizer and speakers’ bureau fees from Amgen, Biologics, AstraZeneca, New Bridge, and Pfizer. P.P.A. has received research funding from the Melanoma Research Alliance. R.S.O. has received honoraria and speakers’ bureau payments from Recordati. He has also received travel expenses from Beckman Coulter and Recordati. A.A.H., H.A-M., A.A.M., H.R., and R.M.S. have no conflicts to report.
Figures
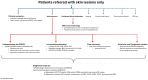

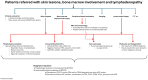
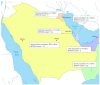
References
-
- Khoury J.D., Solary E., Abla O., Akkari Y., Alaggio R., Apperley J.F., Bejar R., Berti E., Busque L., Chan J.K.C., et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. - DOI - PMC - PubMed
-
- Ohgami R.S., Aung P.P., Gru A.A., Hussaini M., Singh K., Querfeld C., Yao K., Small C., Gollapudi S., Jaye D., et al. An Analysis of the Pathologic Features of Blastic Plasmacytoid Dendritic Cell Neoplasm Based on a Comprehensive Literature Database of Cases. Arch. Pathol. Lab. Med. 2023;147:837–846. doi: 10.5858/arpa.2021-0612-RA. - DOI - PubMed
-
- Garnache-Ottou F., Vidal C., Biichle S., Renosi F., Poret E., Pagadoy M., Desmarets M., Roggy A., Seilles E., Soret L., et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238–4251. doi: 10.1182/bloodadvances.2019000647. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

