Diagnosis and Management of Skin Toxicities in Systemic Treatment of Genitourinary Cancers
- PMID: 39858032
- PMCID: PMC11763385
- DOI: 10.3390/cancers17020251
Diagnosis and Management of Skin Toxicities in Systemic Treatment of Genitourinary Cancers
Abstract
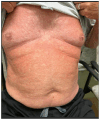
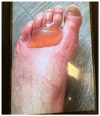
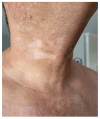
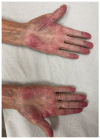
The landscape of available therapeutic options for treatment of genitourinary (GU) cancers is expanding dramatically. Many of these treatments have distinct, sometimes severe, skin toxicities including morbilliform, bullous, pustular, lichenoid, eczematous, psoriasiform, and palmoplantar eruptions. Pruritus and skin pigmentation changes have also been noted. This review aims to synthesize dermatologic events observed with antibody drug conjugates, poly (ADP-ribose) polymerase (PARP) inhibitors, androgen receptor pathway inhibitors, tyrosine kinase inhibitors, immune checkpoint inhibitors, and the combination of these agents used for the treatment of GU cancers. It provides a guide on diagnosis and initial management of these rashes for medical oncologists.
Keywords: Stevens–Johnson syndrome; genitourinary cancers; immunotherapy; prostate cancer; rash; renal cell carcinoma; skin disorders; urothelial carcinoma.
Conflict of interest statement
A.B. reports honorarium from Jansen. A.F. reports no relevant conflicts of interest. C.M.C. reports honoraria from Bayer, EMD Serono, Pfizer, Janssen, Avanced Acel, Ipsen, Astellas Seagen, Eisai, Merck, Bayer, BMS, Tolmar; Travel grant from Janssen, EMD Serono. D.B. reports honorarium for consultancy and speaker fees: AstraZeneca, Ipsen, Merck, Pfizer, AbbVie, Amgen, Janssen, Knight Therapeutics, Eisai, EMD Serono, Bayer and research funding from Ipsen, Knight Therapeutics. D.C. reports honoraria from: Pfizer. D.J.T. reports honoraria from AbbVie, Medexa, Novartis, Pfizer, and L’Oréal; consultancy fees from AbbVie, UCB Biopharma, Kyowa Kirin, Recordati; and a role as a sub-investigator for clinical trials sponsored by Amgen, AbbVie, and Galderma. L.C. reports no relevant conflicts of interest. M.F.S. reports honorarium from AstraZeneca, Pfizer, Novartis, Merks, Seagen, Roche, Gilead, Lilly. M.G.K. reports honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Amgen, Arcutis, Bausch, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Recordati, Sanofi-Genzyme, UCB Biopharma; and consulting fees from AbbVie, Arcutis, Bausch, Eli Lilly, Invyte, Janssen, Leo, Novartis, Pfizer, Recordati, Sanofi-Genzyme, Therako, UCB Biopharma. M.N.R. reports honoraria from: Pfizer, Ipsen, EMD Serono, Merck, Bayer. M.O. reports consulting fees from: Janssen, Bayer, Astra-Zeneca, BMS, Merck, Novartis, Pfizer, and EMD Serrono, as well as research funding from Astra-Zeneca, Bayer, and BMS. R.O. reports no relevant conflicts of interest.
Figures
References
-
- FDA Adverse Reporting Sytem (FAERS) Public Dashboard. [(accessed on 12 October 2024)]; Available online: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet....
-
- Bolognia J.L., Schaffer J.C.L., editors. Dermatology. Elsevier; Amsterdam, The Netherlands: 2018. Erythemas and Purpuras: Drug Reactions; pp. 348–375.
-
- Bolognia J., Schaffer J., Cerroni L. Papulosquamous and Eczematous Dermatoses: Atopic Dermatitis. In: McAleer M., Irvine A., editors. Dermatology. Elsevier; Amsterdam, The Netherlands: 2018. pp. 210–231.
Publication types
LinkOut - more resources
Full Text Sources