Comprehensive Review of Early and Late Toxicities in CAR T-Cell Therapy and Bispecific Antibody Treatments for Hematologic Malignancies
- PMID: 39858064
- PMCID: PMC11764151
- DOI: 10.3390/cancers17020282
Comprehensive Review of Early and Late Toxicities in CAR T-Cell Therapy and Bispecific Antibody Treatments for Hematologic Malignancies
Abstract
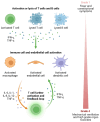
Chimeric antigen receptor T-cell (or CAR-T) therapy and bispecific antibodies (BsAbs) have revolutionized the treatment of hematologic malignancies, offering new options for relapsed or refractory cases. However, these therapies carry risks of early complications, such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), and delayed issues like graft-versus-host disease (GVHD), infections, and secondary cancers. Effective management requires early diagnosis using advanced biomarkers and imaging, along with prompt interventions involving immunosuppressants, corticosteroids, and cytokine inhibitors. A multidisciplinary approach is essential, integrating hematologists, oncologists, and infectious disease specialists, with emerging strategies like targeted biologics and personalized medicine showing promise in balancing efficacy with toxicity management. Ongoing research is critical to refine diagnostics and treatments, ensuring that these therapies not only extend survival but also improve patients' quality of life. This review provides critical insights for healthcare professionals to quickly recognize and treat complications of CAR-T and BsAbs therapies. By focusing on early detection through biomarkers and imaging and outlining timely therapeutic interventions, it aims to equip the multidisciplinary care team with the knowledge necessary to manage the challenges of these advanced treatments effectively, ultimately optimizing patient outcomes.
Keywords: CAR-T therapy; bispecific T-cell engager therapies; complications; hematologic malignancies.
Conflict of interest statement
A.B., B.A., C.M., L.Z., and C.C. declare no conflicts of interest. A.I.: Consultancy, Honoraria, and/or Speaker Bureau: TG Therapeutics and AstraZeneca; Advisory Board: Secura Bio.
Figures
References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Budde L.E., Sehn L.H., Matasar M., Schuster S.J., Assouline S., Giri P., Kuruvilla J., Canales M., Dietrich S., Fay K., et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23:1055–1065. doi: 10.1016/S1470-2045(22)00335-7. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources