Detection and Characterization of Circulating Tumor Cells in Colorectal Cancer Patients via Epithelial-Mesenchymal Transition Markers
- PMID: 39858085
- PMCID: PMC11763958
- DOI: 10.3390/cancers17020303
Detection and Characterization of Circulating Tumor Cells in Colorectal Cancer Patients via Epithelial-Mesenchymal Transition Markers
Abstract
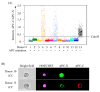
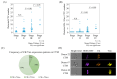
Background/Objectives: Liquid biopsy methods have gained prominence as minimally invasive tools to improve cancer treatment outcomes. Circulating tumor cells (CTCs) offer valuable insights into both primary and metastatic lesions. However, validating the CTC test results requires confirmation that the detected cells originate from cancer tissue. While studies have identified CTCs in colorectal cancer (CRC) patients using molecular markers, simultaneous validation of their cancer tissue origin remains unexplored. Methods: This study introduces a simple approach to detect adenomatous polyposis coli (APC) gene abnormalities alongside established CTC markers using a molecular imaging flow cytometer (MI-FCM). Given that APC gene abnormalities occur in 60-70% of CRC patients, their detection serves as strong evidence of cancer origin. Results: Our method achieved 92% concordance with DNA sequence analysis of tumor-derived cells. In a proof-of-concept study using 5 mL of whole blood from CRC patients, we observed a high frequency of cells exhibiting APC abnormalities, cytokeratin (CK), and vimentin (Vim) expression. Extending the study to 80 CRC patients across pathological stages I-IV confirmed CK and Vim as valid CTC markers. Three distinct cell populations were identified in blood: CK+/Vim-, CK+/Vim+, and CK-/Vim+. CTC number and frequency increased progressively with cancer stage. Conclusions: This is the first report demonstrating CK and Vim as effective markers for direct CTC detection in CRC patients. Our findings provide evidence-based validation of CTC markers, offering new insights and advancing approaches for patient care.
Keywords: adenomatous polyposis coli; circulating tumor cells; colorectal cancer; liquid biopsy.
Conflict of interest statement
Y.T., Y.I., N.E., A.K., K.S., S.I. and Y.M. are employees of Sysmex Corporation. Sysmex Corporation has provided salaries for all authors and covered the research costs. However, Sysmex Corporation had no role in the design, execution, interpretation, or writing of the study.
Figures



References
-
- Ross A.A., Cooper B.W., Lazarus H.M., Mackay W., Moss T.J., Ciobanu N., Tallman M.S., Kennedy M.J., Davidson N.E., Sweet D. Detection and viability of tumor cells in peripheral blood stem cell collections from breast cancer patients using immunocytochemical and clonogenic assay techniques. Blood. 1993;82:2605–2610. doi: 10.1182/blood.V82.9.2605.2605. - DOI - PubMed
-
- Ghossein R.A., Bhattacharya S., Rosai J. Molecular detection of micrometastases and circulating tumor cells in solid tumors. Clin. Cancer Res. 1999;5:1950–1960. - PubMed
-
- Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G.J., Uhr J.W., Terstappen L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

