Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (r Fg Abl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells
- PMID: 39858178
- PMCID: PMC11758316
- DOI: 10.3390/ani15020179
Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (r Fg Abl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells
Erratum in
-
Correction: Zhao et al. Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals 2025, 15, 179.Animals (Basel). 2025 Feb 14;15(4):553. doi: 10.3390/ani15040553. Animals (Basel). 2025. PMID: 39989403 Free PMC article.
Abstract
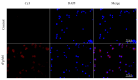
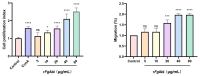
Fasciola gigantica can modulate host immune mechanisms through excretory-secretory products (ESP). As one of the components of ESP, it is unknown whether Abelson tyrosine protein kinase (Abl) is involved in parasite-host immune interaction. To investigate the immunoregulatory function of Abl in Fasciola gigantica, we cloned and expressed the Fasciola gigantica Abl protein and assessed its effect on specific immune functions of buffalo peripheral blood mononuclear cells (PBMCs). Recombinant F. gigantica Abelson tyrosine protein kinase (rFgAbl) was expressed in Escherichia coli. Western blot analysis was performed to assess the reactivity of anti-rFgAbl antibodies with rFgAbl, serum from F. gigantica-infected buffalo, and excretion and secretion products of F. gigantica. Immunohistochemical analysis was conducted to determine the localization of FgAbl in tissues from larval stages and adult worms of F. gigantica. Furthermore, immunofluorescence analysis was utilized to evaluate the binding ability of the rFgAbl protein to buffalo peripheral blood mononuclear cells (PBMCs), as well as to investigate the effects of varying concentrations of rFgAbl protein (5, 10, 20, 40, and 80 μg/mL) on the functional responses of PBMCs. Anti-rFgAbl antibodies specifically recognize rFgAbl, serum from buffalo infected with F. gigantica, and FgESP. rFgAbl is localized in the cecum and capsule of juvenile worms, as well as in the testis and viellaria of adult worms. Additionally, rFgAbl enhances cell proliferation, migration, nitric oxide (NO) production, and phagocytosis, while also increasing the transcription levels of cytokines (IFN-γ, IL-12, TNF-α, IL-4, IL-10, and TGF-β). The results indicate that rFgAbl can influence the immune function of PBMCs. Further investigation into the immunomodulatory properties of the rFgAbl protein will enhance our understanding of the immune interaction mechanisms between trematodes and their hosts.
Keywords: Abelson tyrosine protein kinase; Fasciola gigantica; host–parasite interactions; immune regulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Correction: Zhao et al. Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals 2025, 15, 179.Animals (Basel). 2025 Feb 14;15(4):553. doi: 10.3390/ani15040553. Animals (Basel). 2025. PMID: 39989403 Free PMC article.
-
A recombinant Fasciola gigantica 14-3-3 epsilon protein (rFg14-3-3e) modulates various functions of goat peripheral blood mononuclear cells.Parasit Vectors. 2018 Mar 6;11(1):152. doi: 10.1186/s13071-018-2745-4. Parasit Vectors. 2018. PMID: 29510740 Free PMC article.
-
Immune modulation of buffalo peripheral blood mononuclear cells by two asparaginyl endopeptidases from Fasciola gigantica.Parasit Vectors. 2024 Dec 18;17(1):516. doi: 10.1186/s13071-024-06570-5. Parasit Vectors. 2024. PMID: 39696651 Free PMC article.
-
Reconstruction of the TGF-β signaling pathway of Fasciola gigantica.Parasitol Res. 2023 Dec 14;123(1):51. doi: 10.1007/s00436-023-08064-2. Parasitol Res. 2023. PMID: 38095703 Review.
-
Differential expression of microRNAs and tRNA fragments mediate the adaptation of the liver fluke Fasciola gigantica to its intermediate snail and definitive mammalian hosts.Int J Parasitol. 2021 Apr;51(5):405-414. doi: 10.1016/j.ijpara.2020.10.009. Epub 2021 Jan 26. Int J Parasitol. 2021. PMID: 33513403 Review.
Cited by
-
Correction: Zhao et al. Fasciola gigantica Recombinant Abelson Tyrosine Protein Kinase (rFgAbl) Regulates Various Functions of Buffalo Peripheral Blood Mononuclear Cells. Animals 2025, 15, 179.Animals (Basel). 2025 Feb 14;15(4):553. doi: 10.3390/ani15040553. Animals (Basel). 2025. PMID: 39989403 Free PMC article.
References
-
- Hanna R.E., Moffett D., Forster F.I., Trudgett A.G., Brennan G.P., Fairweather I. Fasciola hepatica: A light and electron microscope study of the ovary and of the development of oocytes within eggs in the uterus provides an insight into reproductive strategy. Vet. Parasitol. 2016;221:93–103. doi: 10.1016/j.vetpar.2016.03.011. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

