Cardiovascular Risk Biomarkers in Women with and Without Polycystic Ovary Syndrome
- PMID: 39858399
- PMCID: PMC11763313
- DOI: 10.3390/biom15010004
Cardiovascular Risk Biomarkers in Women with and Without Polycystic Ovary Syndrome
Abstract
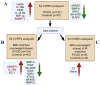
Objective: Polycystic ovary syndrome (PCOS) is a prevalent metabolic disorder with an increased risk for cardiovascular disease (CVD) that is enhanced by obesity. This study sought to determine whether a panel of cardiovascular risk proteins (CVRPs) would be dysregulated in overweight/obese PCOS patients, highlighting potential biomarkers for CVD in PCOS.
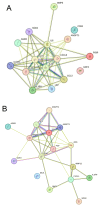
Methods: In this exploratory cross-sectional study, plasma levels of 54 CVRPs were analyzed in women with PCOS (n = 147) and controls (n = 97). CVRPs were measured using the SOMAscan proteomic platform (version 3.1), with significant proteins identified through linear models, regression analysis, and receiver operating characteristic (ROC) analysis. Analysis on BMI-matched subsets of the cohort were undertaken. Functional enrichment and protein-protein interaction analyses elucidated the pathways involved.
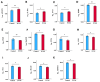
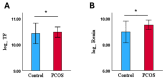
Results: Eleven CVRPs were dysregulated in PCOS (whole set, without matching for body mass index (BMI) or insulin resistance (IR)): leptin, Interleukin-1 receptor antagonist protein (IL-1Ra), polymeric immunoglobulin receptor (PIGR), interleukin-18 receptor (IL-18Ra), C-C motif chemokine 3 (MIP-1a), and angiopoietin-1 (ANGPT1) were upregulated whilst advanced glycosylation end product-specific receptor, soluble (sRAGE), bone morphogenetic protein 6 (BMP6); growth/differentiation factor 2 (GDF2), superoxide dismutase [Mn] mitochondrial (MnSOD), and SLAM family member 5 (SLAF5) were downregulated versus the controls. In BMI-matched (overweight/obese, BMI ≥ 26 kg/m2) subset analysis, six CVRPs were common to the whole set: ANGPT1 and IL-1Ra were upregulated; and sRAGE, BMP6, GDF2, and Mn-SOD were downregulated. In addition, lymphotactin (XCL1) was upregulated and placenta growth factor (PIGF), alpha-L-iduronidase (IDUA), angiopoietin-1 receptor, and soluble (sTie-2) and macrophage metalloelastase (MMP12) were downregulated. A subset analysis of BMI-matched plus insulin resistance (IR)-matched women revealed only upregulation of tissue factor (TF) and renin in PCOS, potentially serving as biomarkers for cardiovascular risk in overweight/obese women with PCOS.
Conclusions: A combination of upregulated obesity-related CVRPs (ANGPT1/IL/1Ra/XCL1) and downregulated cardioprotective proteins (sRAGE/BMP6/Mn-SOD/GDF2) in overweight/obese PCOS women may contribute to the increased risk for CVD. TF and renin upregulation observed in the BMI- and IR-matched limited sample PCOS subgroup indicates their potential risk of CVD.
Keywords: PCOS; biomarkers; cardiovascular risk; polycystic ovary syndrome; proteomics.
Conflict of interest statement
No authors have any conflicts of interest or competing interests to declare.
Figures


References
-
- Salari N., Nankali A., Ghanbari A., Jafarpour S., Ghasemi H., Dokaneheifard S., Mohammadi M. Global prevalence of polycystic ovary syndrome in women worldwide: A comprehensive systematic review and meta-analysis. Arch. Gynecol. Obstet. 2024;310:1303–1314. doi: 10.1007/s00404-024-07607-x. - DOI - PubMed
-
- Diamanti-Kandarakis E., Kouli C.R., Bergiele A.T., Filandra F.A., Tsianateli T.C., Spina G.G., Zapanti E.D., Bartzis M.I. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J. Clin. Endocrinol. Metab. 1999;84:4006–4011. doi: 10.1210/jcem.84.11.6148. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

