NADH Reductive Stress and Its Correlation with Disease Severity in Leigh Syndrome: A Pilot Study Using Patient Fibroblasts and a Mouse Model
- PMID: 39858433
- PMCID: PMC11764390
- DOI: 10.3390/biom15010038
NADH Reductive Stress and Its Correlation with Disease Severity in Leigh Syndrome: A Pilot Study Using Patient Fibroblasts and a Mouse Model
Abstract
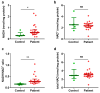
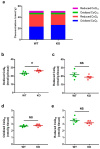
Nicotinamide adenine dinucleotide (NAD) is a critical cofactor in mitochondrial energy production. The NADH/NAD+ ratio, reflecting the balance between NADH (reduced) and NAD+ (oxidized), is a key marker for the severity of mitochondrial diseases. We recently developed a streamlined LC-MS/MS method for the precise measurement of NADH and NAD+. Utilizing this technique, we quantified NADH and NAD+ levels in fibroblasts derived from pediatric patients and in a Leigh syndrome mouse model in which mitochondrial respiratory chain complex I subunit Ndufs4 is knocked out (KO). In patient-derived fibroblasts, NAD+ levels did not differ significantly from those of healthy controls (p = 0.79); however, NADH levels were significantly elevated (p = 0.04), indicating increased NADH reductive stress. This increase, observed despite comparable total NAD(H) levels between the groups, was attributed to elevated NADH levels. Similarly, in the mouse model, NADH levels were significantly increased in the KO group (p = 0.002), further suggesting that NADH elevation drives reductive stress. This precise method for NADH measurement is expected to outperform conventional assays, such as those for lactate, providing a simpler and more reliable means of assessing disease progression.
Keywords: LC-MS/MS; Leigh syndrome; NADH; Ndufs4-KO mice; mitochondrial diseases; reductive stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

