Importance of Modulating Kynurenic Acid Metabolism-Approaches for the Treatment of Dementia
- PMID: 39858468
- PMCID: PMC11764436
- DOI: 10.3390/biom15010074
Importance of Modulating Kynurenic Acid Metabolism-Approaches for the Treatment of Dementia
Abstract
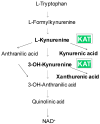
In this article, we focus on kynurenic acid metabolism in neuropsychiatric disorders and the biochemical processes involved in memory and cognitive impairment, followed by different approaches in the fight against dementia. Kynurenic acid-a biochemical part of L-tryptophan catabolism-is synthesized from L-kynurenine by kynurenine aminotransferases. Experimental pharmacological studies have shown that elevated levels of kynurenic acid in the brain are associated with impaired learning and that lowering kynurenic acid levels can improve these symptoms. The discovery of new compounds with the ability to block kynurenine aminotransferases opens new therapeutic avenues for the treatment of memory impairment and dementia. The newly developed Helix pomatia snail model of memory can be used for the assessment of novel pharmacological approaches. Dietary supplementation with natural molecular/herbal extracts, exercise, and physical activity have significant impacts on endogenous pharmacology by reducing kynurenic acid synthesis, and these factors are likely to significantly modulate steady-state biological conditions and delay the negative consequences of aging, including the onset of pathological processes.
Keywords: D-cycloserine; Helix pomatia snail; Jerusalem Balsam; anti-dementia drug; bird droppings; cerebrolysin; dementia; glial depressing factor; herbs; kynurenic acid; memory model; plaque; xanthurenic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Stone T.W. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 1993;45:309–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

