Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe?
- PMID: 39858496
- PMCID: PMC11763651
- DOI: 10.3390/biom15010102
Tetrahydrobiopterin in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Friend or Foe?
Abstract
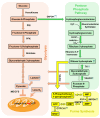
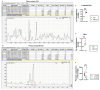
Myalgic Encephalomyelitis or Chronic Fatigue Syndrome (ME/CFS) is a chronic multisystem disease characterized by severe muscle fatigue, pain, dizziness, and brain fog. The two most common symptoms are post-exertional malaise (PEM) and orthostatic intolerance (OI). ME/CFS patients with OI (ME+OI) suffer from dizziness or faintness due to a sudden drop in blood pressure while maintaining an upright posture. Clinical research has demonstrated that patients with OI display severe cardiovascular abnormalities resulting in reduced effective blood flow in the cerebral blood vessels. However, despite intense investigation, it is not known why the effective cerebral blood flow is reduced in OI patients. Based on our recent findings, we observed that tetrahydrobiopterin (BH4) metabolism was highly dysregulated in ME+OI patients. In the current review article, we attempted to summarize our recent findings on BH4 metabolism to shed light on the molecular mechanisms of OI.
Keywords: ME/CFS; autophagy; dihydrobiopterin (BH2); orthostatic intolerance (OI); oxidative stress; pentose phosphate pathway (PPP); tetrahydrobiopterin (BH4).
Conflict of interest statement
A.R., S.B. and C.G.G. are employees of Simmaron Research INC, a 501C non-profit research organization. The authors declare no conflicts of interest.
Figures





References
-
- Kaufman S. Ciba Foundation Symposium 22-Aromatic Amino Acids in the Brain. Wiley Online Library; Hoboken, NJ, USA: 1974. Properties of the pterin-dependent aromatic amino acid hydroxylases; pp. 85–115.
-
- Hopkins F.G. Note on a yellow pigment in butterflies. Nature. 1889;40:335
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

