Control of BKPyV-DNAemia by a Tailored Viro-Immunologic Approach Does Not Lead to BKPyV-Nephropathy Progression and Development of Donor-Specific Antibodies in Pediatric Kidney Transplantation
- PMID: 39858816
- PMCID: PMC11767705
- DOI: 10.3390/microorganisms13010048
Control of BKPyV-DNAemia by a Tailored Viro-Immunologic Approach Does Not Lead to BKPyV-Nephropathy Progression and Development of Donor-Specific Antibodies in Pediatric Kidney Transplantation
Abstract
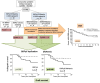
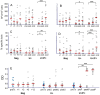
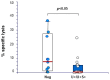
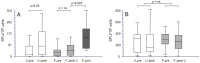
Polyomavirus BK (BKPyV)-associated nephropathy (BKPyV-nephropathy) remains a significant cause of premature kidney allograft failure. In the absence of effective antiviral treatments, current therapeutic approaches rely on immunosuppression (IS) reduction, possibly at the risk of inducing alloimmunity. Therefore, we sought to explore the long-term effects of a tailored viro-immunologic surveillance and treatment program for BKPyV on the development of alloimmunity and kidney graft outcome. Forty-five pediatric kidney transplant recipients were longitudinally monitored for BKPyV replication, virus-specific immunity, and donor-specific HLA antibodies (DSAs). DNAemia developed in 15 patients who were treated with stepwise IS reduction. Among the other 30 patients, 17 developed DNAuria without DNAemia and 13 always resulted as BKPyV-negative. All patients with DNAemia cleared BKPyV after having mounted a virus-specific cellular immune response, and no biopsy-proven BKPyV-nephropathy was observed. The presence of cytotoxic populations directed to the BKPyV Large-T (LT) antigen early after transplantation protected kidney recipients from developing BKPyV replication, and the appearance of LT-specific T cells in viruric patients prevented the development of BKPyV-DNAemia. In our cohort, no significant correlation was observed between BKPyV-DNAemia and the development of DSA and antibody-mediated rejection. However, patients who experienced and cleared BKPyV-DNAemia had a worse allograft survival at a median follow-up of 18.9 years (p = 0.048). These data need to be confirmed in larger cohorts.
Keywords: cellular immunity; donor-specific antibodies; humoral immunity; pediatric kidney transplantation; polyomavirus BK.
Conflict of interest statement
Fondazione Malattie Renali del BambinoThe authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Graf F.E., Hirsch H.H. BK Polyomavirus After Solid Organ and Hematopoietic Cell Transplantation: One Virus –Three Diseases. In: Morris M.I., Kotton C.N., Wolfe C., editors. Emerging Transplant Infections: Clinical Challenges and Implications. Springer International Publishing; Cham, Switzerland: 2020. pp. 1–26.
-
- Kotton C.N., Kamar N., Wojciechowski D., Eder M., Hopfer H., Randhawa P., Sester M., Comoli P., Tedesco Silva H., Knoll G., et al. The Second International Consensus Guidelines on the Management of BK Polyomavirus in Kidney Transplantation. Transplantation. 2024;108:1834–1866. doi: 10.1097/TP.0000000000004976. - DOI - PMC - PubMed
-
- Hirsch H.H., Brennan D.C., Drachenberg C.B., Ginevri F., Gordon J., Limaye A.P., Mihatsch M.J., Nickeleit V., Ramos E., Randhawa P., et al. Polyomavirus-Associated Nephropathy in Renal Transplantation: Interdisciplinary Analyses and Recommendations. Transplantation. 2005;79:1277–1286. doi: 10.1097/01.TP.0000156165.83160.09. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

