Probiotics in the Management of Chronic Bacterial Prostatitis Patients: A Randomized, Double-Blind Trial to Evaluate a Possible Link Between Gut Microbiota Restoring and Symptom Relief
- PMID: 39858898
- PMCID: PMC11767496
- DOI: 10.3390/microorganisms13010130
Probiotics in the Management of Chronic Bacterial Prostatitis Patients: A Randomized, Double-Blind Trial to Evaluate a Possible Link Between Gut Microbiota Restoring and Symptom Relief
Abstract
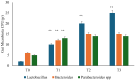
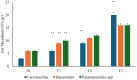
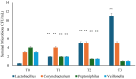
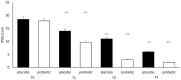
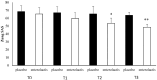
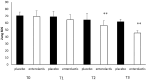
Several studies have suggested that probiotics could play a role in the management of patients with chronic bacterial prostatitis (CBP). In this randomized, placebo-controlled clinical study, we evaluated the efficacy and safety of consumption of probiotics containing human Lactobacillus casei DG® as an add-on treatment in patients with clinical recurrences of CBP, through gut microbiota modification analysis. Enrolled patients with CBP were randomized to receive for 3 months probiotics containing human Lactobacillus casei DG® or placebo following 1 month treatment with ciprofloxacin. During the enrollment and follow-ups, urological examinations analyzed symptoms and quality of life, while microbiological tests analyzed gut and seminal microbiota. During the study, the development of adverse drug reactions was evaluated through the Naranjo scale. Twenty-four patients with CBP were recruited and treated for 3 months with placebo (n. 12) or with Lactobacillus casei DG® (n. 12). Lactobacillus casei DG® induced a significantly (p < 0.01) faster recovery of symptoms than placebo (2 days vs. 8 days) and an increased time free from symptoms (86 days vs. 42 days) without the occurrence of adverse events. In the probiotic group, the appearance of Lactobacilli after 30 days (T1) was higher vs. the placebo group, and a significant reduction in Corynebacterium, Peptoniphilus, Pseudomonas, Veillonella, Staphylococcus, and Streptococcus was also observed. These preliminary data suggest that in patients with CBP, the use of Lactobacillus casei DG after an antimicrobial treatment improves the days free of symptoms and the quality of life, without the development of adverse drug reactions.
Keywords: Lactobacillus casei DG; antibiotic resistance; chronic bacterial prostatitis; microbiota; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources

