From the Cytoplasm into the Nucleus-Hepatitis B Virus Travel and Genome Repair
- PMID: 39858925
- PMCID: PMC11767736
- DOI: 10.3390/microorganisms13010157
From the Cytoplasm into the Nucleus-Hepatitis B Virus Travel and Genome Repair
Abstract
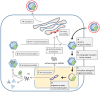
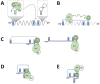
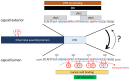
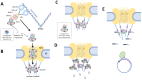
Hepatitis B virus (HBV) is a major global health concern, affecting millions of people worldwide. HBV is part of the hepadnaviridae family and one of the primary causes of acute and chronic liver infections, leading to conditions such as cirrhosis and hepatocellular carcinoma (HCC). Understanding the intracellular transport and genome repair mechanisms of HBV is crucial for developing new drugs, which-in combination with immune modulators-may contribute to potential cures. This review will explore the current knowledge of HBV intracytoplasmic and nuclear transport, as well as genome repair processes, while drawing comparisons to other viruses with nuclear replication.
Keywords: capsid; core protein phosphorylation; genome release; genome repair; hepatitis B virus; intracellular transport; nuclear import; nuclear pore complex (NPC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

