Human Induced Lung Organoids: A Promising Tool for Cystic Fibrosis Drug Screening
- PMID: 39859153
- PMCID: PMC11764749
- DOI: 10.3390/ijms26020437
Human Induced Lung Organoids: A Promising Tool for Cystic Fibrosis Drug Screening
Abstract
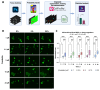
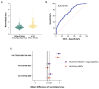
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the CFTR gene. Currently, CFTR modulators are the most effective treatment for CF; however, they may not be suitable for all patients. A representative and convenient in vitro model is needed to screen therapeutic agents under development. This study, on the most common mutation, F508del, investigates the efficacy of human induced pluripotent stem cell-derived lung organoids (hiLOs) from NKX2.1+ lung progenitors and airway basal cells (hiBCs) as a 3D model for CFTR modulator response assessment by a forskolin-induced swelling assay. Weak swelling was observed for hiLOs from NKX2.1+ lung progenitors and hiBCs in response to modulators VX-770/VX-809 and VX-770/VX-661, whereas the VX-770/VX-661/VX-445 combination resulted in the highest swelling response, indicating superior CFTR function restoration. The ROC analysis of the FIS assay results revealed an optimal cutoff of 1.21, with 65.9% sensitivity and 71.8% specificity, and the predictive accuracy of the model was 76.4%. In addition, this study compared the response of hiLOs with the clinical response of patients to therapy and showed similar drug response dynamics. Thus, hiLOs can effectively model the CF pathology and predict patients' specific response to modulators.
Keywords: CFTR modulators; airway basal cells; cystic fibrosis; forskolin-induced swelling assay; human induced pluripotent stem cells; lung organoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

