Synthetic GPR84 Agonists in Colorectal Cancer: Effective in THP-1 Cells but Ineffective in BMDMs and MC38 Mouse Tumor Models
- PMID: 39859206
- PMCID: PMC11764671
- DOI: 10.3390/ijms26020490
Synthetic GPR84 Agonists in Colorectal Cancer: Effective in THP-1 Cells but Ineffective in BMDMs and MC38 Mouse Tumor Models
Abstract
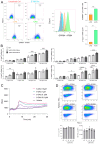
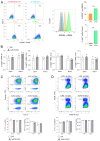
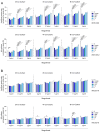
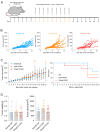
Tumor-associated macrophages (TAMs) in the colorectal cancer (CRC) microenvironment promote tumor progression but can be reprogrammed into a pro-inflammatory state with anti-cancer properties. Activation of the G protein-coupled receptor 84 (GPR84) is associated with pro-inflammatory macrophage polarization, making it a potential target for CRC therapy. This study evaluates the effects of the GPR84 agonists 6-OAU and ZQ-16 on macrophage activation and anti-cancer efficacy. GPR84 expression on THP-1 macrophages and murine BMDMs was analyzed using flow cytometry. Macrophages were treated with 6-OAU or ZQ-16, and pro-inflammatory cytokine levels, reactive oxygen species (ROS) production, and phagocytosis were assessed using qPCR and functional assays. Anti-cancer effects were tested in a subcutaneous MC38 tumor model, with oral or intraperitoneal agonist administration. Pharmacokinetics and compound stability were also evaluated. In THP-1 macrophages, 6-OAU increased pro-inflammatory cytokines and ROS production, with ZQ-16 showing similar effects. However, neither agonist induced pro-inflammatory responses, ROS production, or phagocytosis in murine macrophages. In vivo, both agonists failed to inhibit tumor growth in the MC38 model despite systemic exposure. Current GPR84 agonists lack efficacy in promoting anti-cancer macrophage activity, limiting their potential as CRC therapies.
Keywords: 6-OAU; GPR84; ZQ-16; anti-cancer therapy; colorectal cancer (CRC); immunotherapy; macrophage activation; tumor-associated macrophages (TAMs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

