Pharmacodynamic Mechanisms of Cicadae Periostracum in Parkinson's Disease: A Metabolomics-Based Study
- PMID: 39859260
- PMCID: PMC11764672
- DOI: 10.3390/ijms26020544
Pharmacodynamic Mechanisms of Cicadae Periostracum in Parkinson's Disease: A Metabolomics-Based Study
Abstract
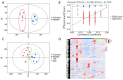
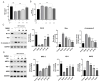
Cicadae Periostracum (CP) is a traditional Chinese animal-derived medicine with the potential to treat Parkinson's disease (PD). This study aims to explore the pharmacodynamic mechanisms of CP against PD-based on metabolomics technology and provide a theoretical basis for developing new anti-PD medicine. First, MPP+-induced SH-SY5Y cells were used to evaluate the anti-PD activity of CP. In the animal study, an MPTP-induced PD mouse model was employed to assess CP's therapeutic effects. Immunofluorescence (IF) staining and Western blotting (WB) were used to evaluate its neuroprotective activity on neurons. A Serum metabolomics analysis was conducted to examine CP's regulatory effects on metabolites and to identify vital metabolic pathways. Finally, cellular experiments were performed to validate the critical pathways. Cellular activity experiments demonstrated that CP mitigates MPP+-induced SH-SY5Y cytotoxicity, inhibits apoptosis, and restores mitochondrial homeostasis. Animal experiments revealed that CP significantly alleviates dyskinesia in PD mice, enhances motor performance, and restores neuronal integrity while reducing α-synuclein (α-syn) aggregation in the striatum (STR), showing its strong anti-PD effect. Metabolomic analysis revealed that CP can significantly improve the metabolic disorders of ten biomarkers that are mainly involved in amino acid metabolism and fatty acid β-oxidation and are closely related to oxidative stress pathways. Finally, pathway verification was performed, and the results show that CP exerted neuroprotective effects against PD through the dual signaling pathways of Bcl-2/Bax/Caspase-3 and Nrf2/HO-1. This study provides a comprehensive strategy for elucidating the mechanisms by which CP exerts its therapeutic effects against PD, highlighting its potential in developing anti-PD drugs.
Keywords: Cicadae Periostracum; Parkinson’s disease; mechanisms of action; metabolomics; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Astragenol alleviates neuroinflammation and improves Parkinson's symptoms through amino acid metabolism pathway and inhibition of ferroptosis.J Ethnopharmacol. 2025 May 28;348:119896. doi: 10.1016/j.jep.2025.119896. Epub 2025 Apr 28. J Ethnopharmacol. 2025. PMID: 40306495
-
Gintonin Mitigates MPTP-Induced Loss of Nigrostriatal Dopaminergic Neurons and Accumulation of α-Synuclein via the Nrf2/HO-1 Pathway.Mol Neurobiol. 2019 Jan;56(1):39-55. doi: 10.1007/s12035-018-1020-1. Epub 2018 Apr 19. Mol Neurobiol. 2019. Retraction in: Mol Neurobiol. 2024 Jul;61(7):4926. doi: 10.1007/s12035-024-04175-8. PMID: 29675576 Retracted.
-
Evaluation of Potential Neuroprotective Effects of Vanillin Against MPP+/MPTP-Induced Dysregulation of Dopaminergic Regulatory Mechanisms in SH-SY5Y Cells and a Mouse Model of Parkinson's Disease.Mol Neurobiol. 2023 Aug;60(8):4693-4715. doi: 10.1007/s12035-023-03358-z. Epub 2023 May 5. Mol Neurobiol. 2023. PMID: 37145378
-
Role and mechanism of molecular hydrogen in the treatment of Parkinson's diseases.Front Neurosci. 2025 Apr 23;19:1576773. doi: 10.3389/fnins.2025.1576773. eCollection 2025. Front Neurosci. 2025. PMID: 40336538 Free PMC article. Review.
-
Metabolomics in Parkinson's Disease and Correlation with Disease State.Metabolites. 2025 Mar 18;15(3):208. doi: 10.3390/metabo15030208. Metabolites. 2025. PMID: 40137172 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

