The Type III Intermediate Filament Protein Peripherin Regulates Lysosomal Degradation Activity and Autophagy
- PMID: 39859265
- PMCID: PMC11766092
- DOI: 10.3390/ijms26020549
The Type III Intermediate Filament Protein Peripherin Regulates Lysosomal Degradation Activity and Autophagy
Abstract
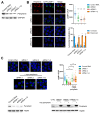
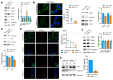
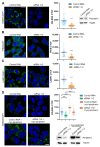
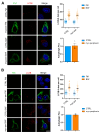
Peripherin belongs to heterogeneous class III of intermediate filaments, and it is the only intermediate filament protein selectively expressed in the neurons of the peripheral nervous system. It has been previously discovered that peripherin interacts with proteins important for the endo-lysosomal system and for the transport to late endosomes and lysosomes, such as RAB7A and AP-3, although little is known about its role in the endocytic pathway. Here, we show that peripherin silencing affects lysosomal abundance but also positioning, causing the redistribution of lysosomes from the perinuclear area to the cell periphery. Moreover, peripherin silencing affects lysosomal activity, inhibiting EGFR degradation and the degradation of a fluorogenic substrate for proteases. Furthermore, we demonstrate that peripherin silencing affects lysosomal biogenesis by reducing the TFEB and TFE3 contents. Finally, in peripherin-depleted cells, the autophagic flux is strongly inhibited. Therefore, these data indicate that peripherin has an important role in regulating lysosomal biogenesis, and positioning and functions of lysosomes, affecting both the endocytic and autophagic pathways. Considering that peripherin is the most abundant intermediate filament protein of peripheral neurons, its dysregulation, affecting its functions, could be involved in the onset of several neurodegenerative diseases of the peripheral nervous system characterized by alterations in the endocytic and/or autophagic pathways.
Keywords: autophagy; cytoskeleton; intermediate filaments; lysosome; peripherin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Role of the Intermediate Filament Protein Peripherin in Health and Disease.Int J Mol Sci. 2022 Dec 6;23(23):15416. doi: 10.3390/ijms232315416. Int J Mol Sci. 2022. PMID: 36499746 Free PMC article. Review.
-
Trigonochinene E promotes lysosomal biogenesis and enhances autophagy via TFEB/TFE3 in human degenerative NP cells against oxidative stress.Phytomedicine. 2023 Apr;112:154720. doi: 10.1016/j.phymed.2023.154720. Epub 2023 Feb 18. Phytomedicine. 2023. PMID: 36868108
-
Impaired TFEB-mediated lysosomal biogenesis promotes the development of pancreatitis in mice and is associated with human pancreatitis.Autophagy. 2019 Nov;15(11):1954-1969. doi: 10.1080/15548627.2019.1596486. Epub 2019 Mar 30. Autophagy. 2019. PMID: 30894069 Free PMC article.
-
Enhancing lysosomal biogenesis and autophagic flux by activating the transcription factor EB protects against cadmium-induced neurotoxicity.Sci Rep. 2017 Feb 27;7:43466. doi: 10.1038/srep43466. Sci Rep. 2017. PMID: 28240313 Free PMC article.
-
Regulation of lysosomes in skeletal muscle during exercise, disuse and aging.Free Radic Biol Med. 2024 Nov 20;225:323-332. doi: 10.1016/j.freeradbiomed.2024.09.028. Epub 2024 Sep 25. Free Radic Biol Med. 2024. PMID: 39332541 Review.
Cited by
-
Targeting autophagy in astrocytes: a potential for neurodegenerative disease intervention.Front Cell Neurosci. 2025 Apr 28;19:1584767. doi: 10.3389/fncel.2025.1584767. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40357169 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

