Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience
- PMID: 39859325
- PMCID: PMC11766022
- DOI: 10.3390/ijms26020611
Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience
Abstract
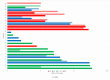
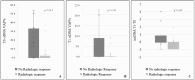
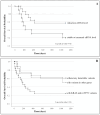
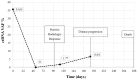
The expression level of Programmed Death-Ligand 1 (PD-L1) determined by the immunohistochemical method is currently approved to test the potential efficacy of immune-checkpoint inhibitors and to candidate patients with Non-Small Cell Lung Cancer (NSCLC) for treatment with immunotherapeutic drugs. As part of the CORELAB (New prediCtivebiOmaRkers of activity and Efficacy of immune checkpoint inhibitors in advanced non-small cell Lung cArcinoma) project, aimed at identifying new predictive and prognostic biomarkers in NSCLC patients receiving immunotherapeutic drugs, we investigated the role of circulating tumor DNA (ctDNA) molecular characterization as an additional predictive biomarker. We analyzed plasma ctDNA by targeted Next Generation Sequencing in a subset of 50 patients at different time points. ctDNA content was inversely correlated with the clinical outcome both at a baseline and after 2 months of treatment. OS was significantly higher in patients with ≥50% ctDNA reduction. TP53 and KRAS were the most frequently mutated genes, and patients with KRAS and/or TP53 mutations showed worse outcomes than patients without detectable variants or with mutations in other genes. Fewer common variants were found in BRAF, EGFR, MAP2K1, MET, NRAS, and PIK3CA genes. Our data demonstrated that molecular characterization of ctDNA and also its quantitative evaluation could serve as a dynamic, real-time prognostic, and predictive biomarker, enabling regular molecular monitoring of therapy efficacy in support of other medical examinations.
Keywords: NGS; NSCLC; PD-L1; immunotherapy; liquid biopsy; prognostic biomarkers.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Gulley J.L., Spigel D., Kelly K., Chandler J.C., Rajan A., Hassan R., Wong D.J.L., Leach J., Edenfield W.J., Wang D., et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J. Clin. Oncol. 2015;33((Suppl. 15)):8034. doi: 10.1200/jco.2015.33.15_suppl.8034. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

