Olaparib Combined with DDR Inhibitors Effectively Prevents EMT and Affects miRNA Regulation in TP53-Mutated Epithelial Ovarian Cancer Cell Lines
- PMID: 39859407
- PMCID: PMC11766100
- DOI: 10.3390/ijms26020693
Olaparib Combined with DDR Inhibitors Effectively Prevents EMT and Affects miRNA Regulation in TP53-Mutated Epithelial Ovarian Cancer Cell Lines
Abstract
Epithelial ovarian cancer (EOC) remains a leading cause of gynecologic cancer mortality. Despite advances in treatment, metastatic progression and resistance to standard therapies significantly worsen patient outcomes. Epithelial-mesenchymal transition (EMT) is a critical process in metastasis, enabling cancer cells to gain invasive and migratory capabilities, often driven by changing miRNA expression involved in the regulation of pathological processes like drug resistance. Targeted therapies like PARP inhibitors (PARPi) have improved outcomes, particularly in BRCA-mutated and DNA repair-deficient tumors; however, resistance and limited efficacy in advanced stages remain challenges. Recent studies highlight the potential synergy of PARPi with DNA damage response (DDR) inhibitors, such as ATR and CHK1 inhibitors, which disrupt cancer cell survival pathways under stress. This study investigated the combined effects of olaparib with ATR and CHK1 inhibitors (ATRi and CHK1i) on migration, invasion, and EMT-related protein expression and miRNA expression in ovarian cancer cell lines OV-90 and SKOV-3. The results demonstrated enhanced cytotoxicity, inhibition of migration and invasion, and modulation of miRNAs linked to metastasis. These findings suggest that combination therapies targeting DNA repair and cell cycle pathways may offer a novel, more effective approach to managing advanced EOC and reducing metastatic spread.
Keywords: DDR inhibitors; EMT; PARPi; miRNA; ovarian cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
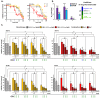
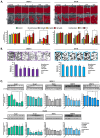


References
-
- Lungchukiet P., Sun Y., Kasiappan R., Quarni W., Nicosia S.V., Zhang X., Bai W. Suppression of epithelial ovarian cancer invasion into the omentum by 1alpha,25-dihydroxyvitamin D3 and its receptor. J. Steroid Biochem. Mol. Biol. 2015;148:138–147. doi: 10.1016/j.jsbmb.2014.11.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

