Utilization of Cannabidiol in Post-Organ-Transplant Care
- PMID: 39859413
- PMCID: PMC11765766
- DOI: 10.3390/ijms26020699
Utilization of Cannabidiol in Post-Organ-Transplant Care
Abstract
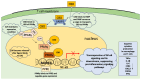
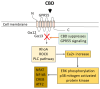
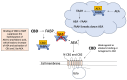
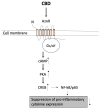
Cannabidiol (CBD) is one of the major phytochemical constituents of cannabis, Cannabis sativa, widely recognized for its therapeutic potential. While cannabis has been utilized for medicinal purposes since ancient times, its psychoactive and addictive properties led to its prohibition in 1937, with only the medical use being reauthorized in 1998. Unlike tetrahydrocannabinol (THC), CBD lacks psychoactive and addictive properties, yet the name that suggests its association with cannabis has significantly contributed to its public visibility. CBD exhibits diverse pharmacological properties, most notably anti-inflammatory effects. Additionally, it interacts with key drug-metabolizing enzyme families, including cytochrome P450 (CYP) and uridine 5'-diphospho-glucuronosyltransferase (UGT), which mediate phase I and phase II metabolism, respectively. By binding to these enzymes, CBD can inhibit the metabolism of co-administered drugs, which can potentially enhance their toxicity or therapeutic effects. Mild to moderate adverse events associated with CBD use have been reported. Advances in chemical formulation techniques have recently enabled strategies to minimize these effects. This review provides an overview of CBD, covering its historical background, recent clinical trials, adverse event profiles, and interactions with molecular targets such as receptors, channels, and enzymes. We particularly emphasize the mechanisms underlying its anti-inflammatory effects and interaction with drugs relevant to organ transplantation. Finally, we explore recent progress in the chemical formulation of CBD in order to enhance its bioavailability, which will enable decreasing the dose to use and increase its safety and efficacy.
Keywords: adverse events; cannabidiol; cannabis plant chemical constituent; chemical formulation; cytochrome P450; drug–drug interaction; inflammation; organ transplant; pharmacodynamics; pharmacokinetics.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study and in the writing of the manuscript.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

