Acute Severe Hypoxia Decreases Mitochondrial Chain Complex II Respiration in Human Peripheral Blood Mononuclear Cells
- PMID: 39859418
- PMCID: PMC11765662
- DOI: 10.3390/ijms26020705
Acute Severe Hypoxia Decreases Mitochondrial Chain Complex II Respiration in Human Peripheral Blood Mononuclear Cells
Abstract
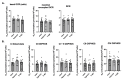
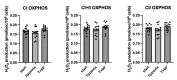
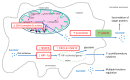
Peripheral blood mononuclear cells' (PBMCs) mitochondrial respiration is impaired and likely involved in myocardial injury and heart failure pathophysiology, but its response to acute and severe hypoxia, often associated with such diseases, is largely unknown in humans. We therefore determined the effects of acute hypoxia on PBMC mitochondrial respiration and ROS production in healthy volunteers exposed to controlled oxygen reduction, achieving an inspired oxygen fraction of 10.5%. We also investigated potential relationships with gene expression of key biomarkers of hypoxia, succinate and inflammation, as hypoxia and inflammation share common mechanisms involved in cardiovascular disease. Unlike global mitochondrial respiration, hypoxemia with a spO2 ≤ 80% significantly reduced PBMC complex II respiration (from 6.5 ± 1.2 to 3.1 ± 0.5 pmol/s/106 cell, p = 0.04). Complex II activity correlated positively with spO2 (r = 0.63, p = 0.02) and inversely correlated with the succinate receptor SUCNR1 (r = -0.68), the alpha-subunit of the hypoxia-inducible factor (HIF-1α, r = -0.61), the chemokine ligand-9 (r = -0.68) and interferon-stimulated gene 15 (r = -0.75). In conclusion, severe hypoxia specifically impairs complex II respiration in association with succinate, inflammation and HIF-1α pathway interactions in human PBMCs. These results support further studies investigating whether modulation of complex II activity might modify the inflammatory and metabolic alterations observed in heart failure.
Keywords: PBMCs; complex II; hypoxia; inflammation; mitochondrial respiration; reactive oxygen species; succinate dehydrogenase.
Conflict of interest statement
All authors have no conflicts of interest with this study.
Figures
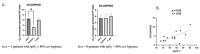

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

