Botulinum Toxin: A Comprehensive Review of Its Molecular Architecture and Mechanistic Action
- PMID: 39859491
- PMCID: PMC11766063
- DOI: 10.3390/ijms26020777
Botulinum Toxin: A Comprehensive Review of Its Molecular Architecture and Mechanistic Action
Abstract
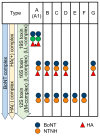
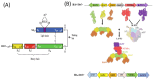
Botulinum toxin (BoNT), the most potent substance known to humans, likely evolved not to kill but to serve other biological purposes. While its use in cosmetic applications is well known, its medical utility has become increasingly significant due to the intricacies of its structure and function. The toxin's structural complexity enables it to target specific cellular processes with remarkable precision, making it an invaluable tool in both basic and applied biomedical research. BoNT's potency stems from its unique structural features, which include domains responsible for receptor recognition, membrane binding, internalization, and enzymatic cleavage. This division of labor within the toxin's structure allows it to specifically recognize and interact with synaptic proteins, leading to precise cleavage at targeted sites within neurons. The toxin's mechanism of action involves a multi-step process: recognition, binding, and catalysis, ultimately blocking neurotransmitter release by cleaving proteins like SNAP-25, VAMP, and syntaxin. This disruption in synaptic vesicle fusion causes paralysis, typically in peripheral neurons. However, emerging evidence suggests that BoNT also affects the central nervous system (CNS), influencing presynaptic functions and distant neuronal systems. The evolutionary history of BoNT reveals that its neurotoxic properties likely provided a selective advantage in certain ecological contexts. Interestingly, the very features that make BoNT a potent toxin also enable its therapeutic applications, offering precision in treating neurological disorders like dystonia, spasticity, and chronic pain. In this review, we highlight the toxin's structural, functional, and evolutionary aspects, explore its clinical uses, and identify key research gaps, such as BoNT's central effects and its long-term cellular impact. A clear understanding of these aspects could facilitate the representation of BoNT as a unique scientific paradigm for studying neuronal processes and developing targeted therapeutic strategies.
Keywords: Bacillus sp.; BoNT/NTNHA-like component A; Clostridiaceae; Clostridium family; E-cadherin; SNAP-23; SNAP-25; SNARE proteins; TNF; botulinum neurotoxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hill K.K., Smith T.J. Genetic diversity within Clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 2013;364:1–20. - PubMed
-
- Kumar R., Chang T.W., Singh B.R. Evolutionary traits of toxins. In: Goparlakrishnakone P., editor. Biological Toxins and Bioterrorism. Springer; Berlin/Heidelberg, Germany: 2015. - DOI
-
- Sebaihia M., Peck M.W., Minton N.P., Thomson N.R., Holden M.T., Mitchell W.J., Carter A.T., Bentley S.D., Mason D.R., Crossman L., et al. Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall A and comparative analysis of the clostridial genomes. Genome Res. 2007;17:1082–1092. doi: 10.1101/gr.6282807. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

