Analysis of the Transcriptome Provides Insights into the Photosynthate of Maize Response to Salt Stress by 5-Aminolevulinic Acid
- PMID: 39859501
- PMCID: PMC11765576
- DOI: 10.3390/ijms26020786
Analysis of the Transcriptome Provides Insights into the Photosynthate of Maize Response to Salt Stress by 5-Aminolevulinic Acid
Abstract
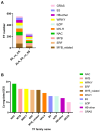
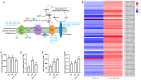
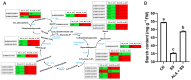
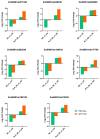
Salt stress is a significant environmental factor that impedes maize growth and yield. Exogenous 5-aminolevulinic acid (ALA) has been shown to mitigate the detrimental effects of various environmental stresses on plants. However, its regulatory role in the photosynthesis mechanisms of maize seedlings under salt stress remains poorly understood. Transcriptome sequencing and physiological index measurements were conducted on the leaves of the "Zhengdan 958" cultivar subjected to three different treatments. Differential expression analysis revealed 4634 differentially expressed genes (DEGs), including key transcription factor (TF) families such as NAC, MYB, WRKY, and MYB-related, across two comparisons (SS_vs_CK and ALA_SS_vs_SS). Significant enrichment was observed in the metabolic pathways related to porphyrin metabolism, photosynthesis-antenna proteins, photosynthesis, and carbon fixation in photosynthetic organisms. ALA treatment modulated the expression of photosynthesis-related genes, increased photosynthetic pigment content, and enhanced the activities of superoxide dismutase (SOD) and catalase (CAT), thereby mitigating the excessive accumulation of reactive oxygen species (ROS). Furthermore, ALA increased starch content under salt stress. These findings establish a foundational understanding of the molecular mechanisms through which ALA regulates photosynthesis under salt stress in maize seedlings. Collectively, exogenous ALA enhances maize's salt tolerance by regulating photosynthesis-related pathways.
Keywords: 5-aminolevulinic acid; Zea mays L.; photosynthesis; salt stress; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Exogenous Melatonin Alleviates NaCl Injury by Influencing Stomatal Morphology, Photosynthetic Performance, and Antioxidant Balance in Maize.Int J Mol Sci. 2024 Sep 19;25(18):10077. doi: 10.3390/ijms251810077. Int J Mol Sci. 2024. PMID: 39337563 Free PMC article.
-
Exogenous 5-aminolevulinic acid enhanced saline-alkali tolerance in pepper seedlings by regulating photosynthesis, oxidative damage, and glutathione metabolism.Plant Cell Rep. 2024 Oct 19;43(11):267. doi: 10.1007/s00299-024-03352-2. Plant Cell Rep. 2024. PMID: 39425750
-
Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress.Sci Rep. 2017 Feb 7;7:42039. doi: 10.1038/srep42039. Sci Rep. 2017. PMID: 28169318 Free PMC article.
-
Advances in deciphering the mechanisms of salt tolerance in Maize.Plant Signal Behav. 2025 Dec;20(1):2479513. doi: 10.1080/15592324.2025.2479513. Epub 2025 Mar 18. Plant Signal Behav. 2025. PMID: 40098499 Free PMC article. Review.
-
5-Aminolevulinic Acid: from pyrrole biosynthetic precursor to multifunctional plant growth regulator.J Plant Physiol. 2025 Jul;310:154524. doi: 10.1016/j.jplph.2025.154524. Epub 2025 May 16. J Plant Physiol. 2025. PMID: 40398357 Review.
Cited by
-
Molecular Mechanisms Underlying Salt Tolerance in Maize: A Combined Transcriptome and Metabolome Analysis.Plants (Basel). 2025 Jul 2;14(13):2031. doi: 10.3390/plants14132031. Plants (Basel). 2025. PMID: 40648040 Free PMC article.
References
-
- Swamy Gowda M.R., Hirtemath C., Singh S., Verma R.S. The Influence of NaCl Salt Stress on the Yield and Quality of the Essential Oil from Two Varieties of Rose-scented Geranium (Pelargonium graveleons L’Hér.) Biochem. Syst. Ecol. 2022;105:104532. doi: 10.1016/j.bse.2022.104532. - DOI
-
- Sarabi B., Ghashghaie J. Evaluating the Physiological and Biochemical Responses of Melon Plants to NaCl Salinity Stress Using Supervised and Unsupervised Statistical Analysis. Plant Stress. 2022;4:100067. doi: 10.1016/j.stress.2022.100067. - DOI
-
- Wang Y., Deng C., Ai P., Cui X., Zhang Z. ALM1, Encoding a Fe-superoxide Dismutase, is Critical for Rice Chloroplast Biogenesis and Drought Stress Response. Crop J. 2021;9:1018–1029. doi: 10.1016/j.cj.2020.09.013. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

