Methylglyoxal-Stimulated Mesothelial Cells Prompted Fibroblast-to-Proto-Myofibroblast Transition
- PMID: 39859527
- PMCID: PMC11766140
- DOI: 10.3390/ijms26020813
Methylglyoxal-Stimulated Mesothelial Cells Prompted Fibroblast-to-Proto-Myofibroblast Transition
Abstract
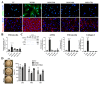
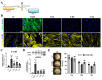
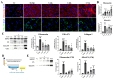
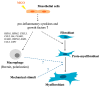
During long-term peritoneal dialysis, peritoneal fibrosis (PF) often happens and results in ultrafiltration failure, which directly leads to the termination of dialysis. The accumulation of extracellular matrix produced from an increasing number of myofibroblasts was a hallmark characteristic of PF. To date, glucose degradation products (GDPs, i.e., methylglyoxal (MGO)) that appeared during the heating and storage of the dialysate are considered to be key components to initiating PF, but how GDPs lead to the activation of myofibroblast in fibrotic peritoneum has not yet been fully elucidated. In this study, mesothelial cell line (MeT-5A) and fibroblast cell line (MRC-5) were used to investigate the transcriptomic and proteomic changes to unveil the underlying mechanism of MGO-induced PF. Our transcriptomic data from the MGO-stimulated mesothelial cells showed upregulation of genes involved in pro-inflammatory, apoptotic, and fibrotic pathways. While no phenotypic changes were noted on fibroblasts after direct MGO, supernatant from MGO-stimulated mesothelial cells promoted fibroblasts to change into proto-myofibroblasts, activated fibroblasts in the first stage toward myofibroblasts. In conclusion, this study showed that MGO-stimulated mesothelial cells promoted fibroblast-to-proto-myofibroblast transition; however, additional involvement of other factors or cells (e.g., macrophages) may be needed to complete the transformation into myofibroblasts.
Keywords: fibroblast-to-myofibroblast; mesothelium; methylglyoxal; peritoneal fibrosis; proto-myofibroblast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

