Challenges in Humoral Immune Response to Adeno-Associated Viruses Determination
- PMID: 39859531
- PMCID: PMC11765838
- DOI: 10.3390/ijms26020816
Challenges in Humoral Immune Response to Adeno-Associated Viruses Determination
Abstract
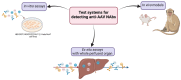
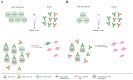
Adeno-associated viruses (AAVs) are non-pathogenic, replication-deficient viruses that have gained widespread attention for their application as gene therapy vectors. While these vectors offer high transduction efficiency and long-term gene expression, the host immune response poses a significant challenge to their clinical success. This review focuses on the obstacles to evaluating the humoral response to AAVs. We discuss the problems with the validation of in vitro tests and the possible approaches to overcome them. Using published data on neutralizing titers of AAV serotypes, we built the first antigenic maps of AAVs in order to visualize the antigenic relationships between varying serotypes.
Keywords: AAV; NHP models; anti-AAV antibodies; antigenic cartography; humanized models; mice models; neutralizing antibodies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Flotte T.R., Carter B.J. Adeno-Associated Viral Vectors. In: Meager A., editor. Gene Therapy Technologies, Applications and Regulations. John, Wiley & Sons; Hoboken, NJ, USA: 1999. pp. 109–125.
-
- Kruzik A., Fetahagic D., Hartlieb B., Dorn S., Koppensteiner H., Horling F.M., Scheiflinger F., Reipert B.M., de la Rosa M. Prevalence of Anti-Adeno-Associated Virus Immune Responses in International Cohorts of Healthy Donors. Mol. Ther. Methods Clin. Dev. 2019;14:126–133. doi: 10.1016/j.omtm.2019.05.014. - DOI - PMC - PubMed
-
- Li C., Narkbunnam N., Samulski R.J., Asokan A., Hu G., Jacobson L.J., Manco-Johnson M.L., Monahan P.E., on behalf of The Joint Outcome Study Investigators Neutralizing Antibodies against Adeno-Associated Virus Examined Prospectively in Pediatric Patients with Hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

