Proteomics Reveals the Response Mechanism of Embryonic Bovine Lung Cells to Mycoplasma bovis Infection
- PMID: 39859536
- PMCID: PMC11765741
- DOI: 10.3390/ijms26020823
Proteomics Reveals the Response Mechanism of Embryonic Bovine Lung Cells to Mycoplasma bovis Infection
Abstract
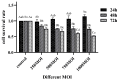
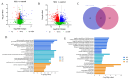
Mycoplasma bovis (M. bovis) has caused huge economic losses to the cattle industry. The interaction between M. bovis and host cells is elucidated by screening and identifying the target protein of M. bovis adhesin on the surface of the host cell membrane. However, the response mechanism of embryonic bovine lung (EBL) cells to M. bovis infection is not yet fully understood. Additionally, it is necessary to further explore whether infection with M. bovis induces oxidative stress and mitochondrial damage in EBL cells. In this study, oxidation reaction, mitochondrial membrane potential, mitochondrial structure, and apoptosis ability of EBL cells infected with M. bovis were assessed at different times (12, 24, 48 h post-infection; hpi). Then, the differential proteomic analysis of M. bovis-infected EBL cells at 12 h and 24 h was performed with uninfected cells as the control. The results showed that M. bovis infection reduced the antioxidant capacity of EBL cells, increased ROS levels, and decreased mitochondrial membrane potential. The mitochondrial membrane of EBL cells was damaged, and the ridge arrangement was disordered after infection by transmission electron microscopy. With the increase in infection time, the mitochondrial matrix partially dissolved and spilled. The apoptosis rate of EBL cells increased with the increase in infection time of M. bovis. Furthermore, proteomic analysis identified 268 and 2061 differentially expressed proteins (DEPs) at 12 hpi and 24 hpi, respectively, compared with the uninfected cells. According to GO analysis, these DEPs were involved in the mitosis and negative regulation of cell growth. Additionally, the Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis indicated the following pathways were linked to mitochondrial damage or cell growth regulation, including glycolysis/gluconeogenesis, pentose phosphate pathway, oxidative phosphorylation, AMPK, cGMP-PKG, cAMP, calcium, Wnt, Phospholipase D, apoptosis, MAPK, cell cycle, Ras, PI3K-Akt, mTOR, HIF-1. PPI results indicated that YWHAZ, PIK3CA, HSP90AB1, RAP1A, TXN, RAF1, MAPK1, PKM, PGK1, and GAPDH might be involved in mitochondrial pathway apoptosis induced by M. bovis infection. This study offers helpful data toward understanding the response of mitochondria of EBL cells to M. bovis infection.
Keywords: Mycoplasma bovis; embryonic bovine lung cells; mitochondrial damage; proteomics; signaling pathway.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Liu S., Li Z., Lan S., Hao H., Jin X., Liang J., Baz A.A., Yan X., Gao P., Chen S., et al. LppA is a novel plasminogen receptor of Mycoplasma bovis that contributes to adhesion by binding the host extracellular matrix and Annexin A2. Vet. Res. 2023;54:107. doi: 10.1186/s13567-023-01242-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

