Lidocaine Enhanced Antitumor Efficacy and Relieved Chemotherapy-Induced Hyperalgesia in Mice with Metastatic Gastric Cancer
- PMID: 39859541
- PMCID: PMC11766172
- DOI: 10.3390/ijms26020828
Lidocaine Enhanced Antitumor Efficacy and Relieved Chemotherapy-Induced Hyperalgesia in Mice with Metastatic Gastric Cancer
Abstract
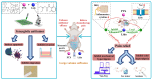
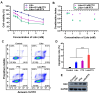
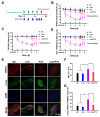
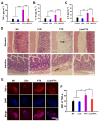
With the widespread use of lidocaine for pain control in cancer therapy, its antitumor activity has attracted considerable attention in recent years. This paper provides a simple strategy of combining lidocaine with chemotherapy drugs for cancer therapy, aiming to relieve chemotherapy-induced pain and achieve stronger antitumor efficacy. However, there is still a lack of substantial pre-clinical evidence for the efficacy and related mechanisms of such combinations, obstructing their potential clinical application. In this study, we propose intraperitoneal chemotherapy (IPC) against gastric cancer (GC) as an ideal scenario to evaluate the efficacy of a lidocaine/paclitaxel combination. Firstly, we used human GC cells MKN-45-luc to investigate the antitumor activity and related mechanisms of the lidocaine/paclitaxel combination in vitro. Then, we used C57BL/6 mice with intraperitoneal drug suffusion to evaluate the efficacy of lidocaine to suppress paclitaxel-induced hyperalgesia and related mechanisms. Lastly, in BALB/c tumor-bearing nude mice we evaluated the synergistic antitumor activity and pain-relieving effect of the lidocaine/paclitaxel combination. Our results showed enhanced antitumor activity for the lidocaine/paclitaxel combination, which induced apoptosis, inhibited migration, and the invasion of GC cells in a synergistic manner. In animal models, the lidocaine/paclitaxel combination effectively inhibited growth and peritoneal metastasis of the tumor, resulting in prolonged survival time. Meanwhile, lidocaine showed considerable anti-inflammatory activity alongside its anesthetic effect, which, in combination, effectively relieved hyperalgesia induced by paclitaxel. These results suggested that intraperitoneal suffusion with lidocaine/paclitaxel could be a pain-free IPC formulation with enhanced antitumor activity, which could provide a promising treatment for GC with peritoneal metastasis.
Keywords: chemotherapy-induced hyperalgesia; gastric cancer; intraperitoneal suffusion; local anesthetics; peritoneal metastasis; synergistic antitumor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer.Oncol Rep. 2014 Jul;32(1):89-96. doi: 10.3892/or.2014.3210. Epub 2014 May 23. Oncol Rep. 2014. PMID: 24859429
-
Efficacy of intraperitoneal chemotherapy with paclitaxel targeting peritoneal micrometastasis as revealed by GFP-tagged human gastric cancer cell lines in nude mice.Int J Oncol. 2005 Sep;27(3):637-44. Int J Oncol. 2005. PMID: 16077911
-
Inhibition of nuclear factor-κB enhances the antitumor effect of paclitaxel against gastric cancer with peritoneal dissemination in mice.Dig Dis Sci. 2013 Jan;58(1):123-31. doi: 10.1007/s10620-012-2311-4. Epub 2012 Jul 18. Dig Dis Sci. 2013. PMID: 22806547
-
Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: experience from Singapore and Japan.Gastric Cancer. 2017 Mar;20(Suppl 1):122-127. doi: 10.1007/s10120-016-0660-y. Epub 2016 Oct 20. Gastric Cancer. 2017. PMID: 27766496 Review.
-
Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis.Gastric Cancer. 2017 Mar;20(Suppl 1):111-121. doi: 10.1007/s10120-016-0662-9. Epub 2016 Nov 1. Gastric Cancer. 2017. PMID: 27803990 Review.
Cited by
-
Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine.Int J Mol Sci. 2025 Jul 24;26(15):7137. doi: 10.3390/ijms26157137. Int J Mol Sci. 2025. PMID: 40806270 Free PMC article.
References
-
- Cristea M.C., Frankel P., Synold T., Rivkin S., Lim D., Chung V., Chao J., Wakabayashi M., Paz B., Han E., et al. A phase I trial of intraperitoneal nab-paclitaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity. Cancer Chemother. Pharmacol. 2019;83:589–598. doi: 10.1007/s00280-019-03767-9. - DOI - PMC - PubMed
-
- Cho H., Ryu M.-H., Kim K.-p., Ryoo B.-Y., Park S.R., Kim B.S., Lee I.-S., Kim H.-S., Yoo M.-W., Yook J.H., et al. Phase I/II study of a combination of capecitabine, cisplatin, and intraperitoneal docetaxel (XP ID) in advanced gastric cancer patients with peritoneal metastasis. Gastric Cancer. 2017;20:970–977. doi: 10.1007/s10120-017-0710-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

