De Novo DNM1L Pathogenic Variant Associated with Lethal Encephalocardiomyopathy-Case Report and Literature Review
- PMID: 39859560
- PMCID: PMC11765995
- DOI: 10.3390/ijms26020846
De Novo DNM1L Pathogenic Variant Associated with Lethal Encephalocardiomyopathy-Case Report and Literature Review
Abstract
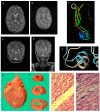
Pathogenic variants in DNM1L, encoding dynamin-like protein-1 (DRP1), cause a lethal encephalopathy. DRP1 defective function results in altered mitochondrial networks, characterized by elongated/spaghetti-like, highly interconnected mitochondria. We validated in yeast the pathogenicity of a de novo DNM1L variant identified by whole exome sequencing performed more than 10 years after the patient's death. Meanwhile, we reviewed the broadness and specificities of DNM1L-related phenotype. The patient, who exhibited developmental delay in her third year, developed a therapy-refractory myoclonic status epilepticus, followed by neurological deterioration with brain atrophy and refractory epilepsy. She died of heart failure due to hypertrophic cardiomyopathy. She was found to be heterozygous for the DNM1L variant (NM_ 012062.5):c.1201G>A, p.(Gly401Ser). We demonstrated its deleterious impact and dominant negative effect by assessing haploid and diploid mutant yeast strains, oxidative growth, oxygen consumption, frequency of petite, and architecture of the mitochondrial network. Structural modeling of p.(Gly401Ser) predicted the interference of the mutant protein in the self-oligomerization of the DRP1 active complex. DNM1L-related phenotypes include static or (early) lethal encephalopathy and neurodevelopmental disorders. In addition, there may be ophthalmological impairment, peripheral neuropathy, ataxia, dystonia, spasticity, myoclonus, and myopathy. The clinical presentations vary depending on mutations in different DRP1 domains. Few pathogenic variants, the p.(Gly401Ser) included, cause an encephalocardiomyopathy with refractory status epilepticus.
Keywords: DNM1L; burst suppression; global developmental regression; hypertrophic cardiomyopathy (HC); lethal encephalocardiomyopathy; refractory status epilepticus (RSE); retrospective post-mortem diagnosis.
Conflict of interest statement
All the authors report no conflicts of interest relevant to the manuscript.
Figures


References
-
- Gerber S., Charif M., Chevrollier A., Chaumette T., Angebault C., Kane M.S., Paris A., Alban J., Quiles M., Delettre C., et al. Mutations in DNM1L, as in OPA1, Result in Dominant Optic Atrophy despite Opposite Effects on Mitochondrial Fusion and Fission. Brain. 2017;140:2586–2596. doi: 10.1093/brain/awx219. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC 13/24 and RC 10/24/Italian Ministry of Health
- Project code PE0000006, Concession Decree No. 1553 of 11/10/2022 adopted by the Italian Ministry of University and Research, CUP D93C22000930002, "A multiscale integrated approach to the study of the nervous system in health and disease" (MNESYS)/Italian Ministry of University and Research funded by the European Union-NextGenerationEU
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

