Navigating the Semiochemical Landscape: Attraction of Subcortical Beetle Communities to Bark Beetle Pheromones, Fungal and Host Tree Volatiles
- PMID: 39859638
- PMCID: PMC11766014
- DOI: 10.3390/insects16010057
Navigating the Semiochemical Landscape: Attraction of Subcortical Beetle Communities to Bark Beetle Pheromones, Fungal and Host Tree Volatiles
Abstract
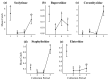
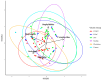
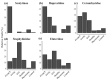
Subcortical beetle communities interact with a wide range of semiochemicals released from different sources, including trees, fungi, and bark beetle pheromones. While the attraction of bark beetles, their insect predators, and competitors to bark beetle pheromones is commonly studied, the attraction of these beetle communities to other sources of semiochemicals remains poorly understood. We tested the attraction of bark and wood-boring beetles and their predators to host stress volatiles, fungal volatiles, and a mountain pine beetle lure in the field. Host stress volatiles were derived from lodgepole pine trees stressed by three fungal symbionts of mountain pine beetle and two common phytopathogens. Our results showed that bark beetles, particularly mountain pine beetles, show a preference for a combination of fungal volatiles, particularly 2-methyl-1-butanol and its lures. Without the addition of lures, 2-methyl-1-butanol was also identified as a key fungal volatile in the attraction of mountain pine beetle competitors from the Cerambycidae and Buprestidae families. Predators in the Elateridae and Staphylinidae families showed attraction to host stress volatiles and the healthy tree volatile profiles. These findings suggest that these semiochemicals warrant further field testing for potential use in monitoring and management of subcortical beetle populations.
Keywords: Atropellis piniphila; Buprestidae; Cerambycidae; Elateridae; Endocronartium harknessii; Grosmannia clavigera; Leptographium longiclavatum; Ophiostoma montium; Staphylinidae; forest health; volatile organic compounds (VOCs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Byers J.A. Chemical ecology of bark beetles in a complex olfactory landscape. In: Lieutier F., Day K.R., Battisti A., Grégoire J.C., Evans H.F., editors. Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis. Springer; Dordrecht, The Netherlands: 2007. - DOI
-
- Erbilgin N., Christiansen E., Krokene P. A host monoterpene influences Ips typographus responses (Coleoptera: Curculionidae, Scolytinae) to its aggregation pheromone: Implications for host colonization of bark beetles. Agric. For. Entomol. 2007;9:135–140. doi: 10.1111/j.1461-9563.2007.00329.x. - DOI
-
- Wallin K.F., Raffa K.F. Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae) Environ. Entomol. 2000;29:442–453. doi: 10.1603/0046-225X-29.3.442. - DOI
LinkOut - more resources
Full Text Sources

