Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis
- PMID: 39859942
- PMCID: PMC11767070
- DOI: 10.3390/life15010002
Investigation of the Effects of Fosfomycin in Kidney Damage Caused by CLP-Induced Sepsis
Abstract
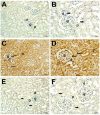
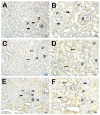
Sepsis, a life-threatening condition characterized by dysregulated host responses to infection, often leads to multi-organ dysfunction, including kidney injury. Kidney damage in sepsis can have severe consequences and is associated with high mortality rates. This study aimed to investigate the potential therapeutic effects of fosfomycin (FOS), a broad-spectrum antibiotic with immunomodulatory properties, on kidney damage induced by cecal ligation and puncture (CLP)-induced sepsis in a rodent model. In total, 24 rats were randomly divided into three groups. Group 1 (n = 8), the healthy control group (C), received a single dose of 0.9% NaCl (saline) solution via an intraperitoneal (i.p.) route. To group 2 (n = 8), the CLP group, CLP-induced sepsis was applied without medication, and a single dose of 0.9% NaCl (saline) solution was applied i.p. before induction. To group 3 (n = 8), the CLP + FOS (500 mg/kg) group, a single dose of 500 mg/kg FOS was administered i.p. before sepsis induction. The effects of fosfomycin on kidney function, histopathological changes, inflammatory markers, oxidative stress, and apoptosis were assessed. In the fosfomycin-treated group, the histological analysis results demonstrated reduction in kidney tissue damage and inflammation. Additionally, fosfomycin attenuated the upregulation of pro-inflammatory cytokines and reduced oxidative stress markers in kidney tissue. Furthermore, fosfomycin treatment was associated with a decrease in apoptotic cell death in the kidney. These findings suggest that fosfomycin may have a protective effect on kidney damage caused by CLP-induced sepsis. The potential mechanisms underlying this protection include the modulation of inflammation, reduction of oxidative stress, and inhibition of apoptosis.
Keywords: CLP; fosfomycin; inflammation; kidney; rat; sepsis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. [(accessed on 28 December 2023)];Lancet. 2020 395:200–211. doi: 10.1016/S0140-6736(19)32989-7. Available online: https://pubmed.ncbi.nlm.nih.gov/31954465/ - DOI - PMC - PubMed
-
- Hoste E.A., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., Edipidis K., Forni L.G., Gomersall C.D., Govil D., et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. [(accessed on 28 December 2023)];Intensive Care Med. 2015 41:1411–1423. doi: 10.1007/s00134-015-3934-7. Available online: https://pubmed.ncbi.nlm.nih.gov/26162677/ - DOI - PubMed
-
- Peerapornratana S., Manrique-Caballero C.L., Gómez H., Kellum J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. [(accessed on 28 December 2023)];Kidney Int. 2019 96:1083–1099. doi: 10.1016/j.kint.2019.05.026. Available online: https://pubmed.ncbi.nlm.nih.gov/31443997/ - DOI - PMC - PubMed
-
- Caraballo C., Jaimes F. Focus: Death: Organ Dysfunction in Sepsis: An Ominous Trajectory From Infection to Death. [(accessed on 28 December 2023)];Yale J. Biol. Med. 2019 92:629. Available online: https://pubmed.ncbi.nlm.nih.gov/31866778/ - PMC - PubMed
-
- Yildiz I.E., Topcu A., Bahceci I., Arpa M., Tumkaya L., Mercantepe T., Batcik S., Yildiz Y. The protective role of fosfomycin in lung injury due to oxidative stress and inflammation caused by sepsis. [(accessed on 28 December 2023)];Life Sci. 2021 279:119662. doi: 10.1016/j.lfs.2021.119662. Available online: https://pubmed.ncbi.nlm.nih.gov/34081989/ - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

