o-Halogenation and -Alkoxylation of Phenylglycine Derivatives by Pd-Mediated C-H Functionalization: Scope and Limitations
- PMID: 39860106
- PMCID: PMC11767792
- DOI: 10.3390/molecules30020236
o-Halogenation and -Alkoxylation of Phenylglycine Derivatives by Pd-Mediated C-H Functionalization: Scope and Limitations
Abstract
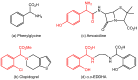
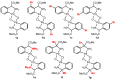
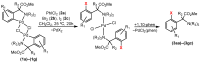
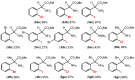
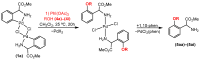
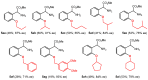
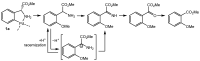
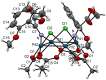
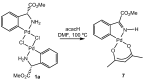
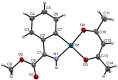
Orthopalladated derivatives from substituted phenylglycines [Pd(μ-Cl)(C6H3R1C(R2)(R3)N(R4)2]2 (1) react with halogenating reagents (PhICl2, Br2, I2) (2) to give the corresponding o-halogenated amino acids C6H3(X)R1C(R2)(R3)N(R4)2 (3). The reaction is general and tolerates a variety of functional groups (R1 to R4) at the aryl ring, the Cα, and the N atom. On the other hand, the reaction of [Pd(μ-Cl)(C6H3R1C(R2)(R3)N(R4)2]2 (1) with PhI(OAc)2 in the presence of a variety of alcohols R5OH (4) gives the o-alkoxylated phenylglycines C6H3(OR5)R1C(R2)(R3)N(R4)2 (5), also as a general process. A partial loss of the enantiomeric excess is observed when the starting phenylglycine is enantiomerically pure, this arising from the formation of bridging azavinylidene (6) and imine intermediate species (7), which were characterized by X-ray diffraction methods.
Keywords: C-H bond activation; alkoxylation; amino acids; halogenation; palladium.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Rogge T., Kaplaneris N., Chatani N., Kim J., Chang S., Punji B., Schafer L.L., Musaev D.G., Wencel-Delord J., Roberts C.A., et al. C–H activation. Nat. Rev. Methods Primers. 2021;1:43. doi: 10.1038/s43586-021-00041-2. - DOI
-
- Rej S., Das A., Chatani N. Strategic evolution in transition metal-catalyzed directed C–H bond activation and future directions. Coord. Chem. Rev. 2021;431:213683. doi: 10.1016/j.ccr.2020.213683. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

