Iron-Catalyzed Cross-Dehydrogenative Coupling
- PMID: 39860120
- PMCID: PMC11767816
- DOI: 10.3390/molecules30020250
Iron-Catalyzed Cross-Dehydrogenative Coupling
Abstract
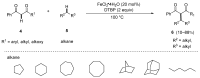
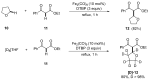
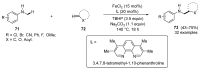
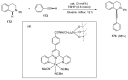
This review highlights significant advances in iron-catalyzed cross-dehydrogenative coupling (CDC), a method pivotal for forming carbon-carbon (C-C) bonds directly from C-H bonds. This technique uses iron-a naturally abundant, inexpensive, and environmentally benign transition metal-as a catalyst to facilitate the coupling of two unfunctionalized C-H bonds. This method stands out for avoiding pre-functionalized substrates, reducing both waste and cost in organic synthesis. The discussion includes a variety of CDC methodologies involving combinations of C(sp3)-H with C(sp3)-H, C(sp3)-H with C(sp2)-H, and C(sp3)-H with C(sp)-H bonds. These methods have been successfully applied in synthesizing complex molecules and pharmaceuticals, highlighting the versatility and efficiency of iron catalysis.
Keywords: C-H bond activation; cross-dehydrogenative coupling; green synthesis; iron catalysis; organic transformations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



























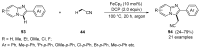

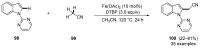






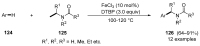



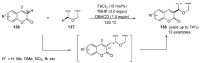


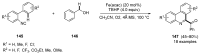



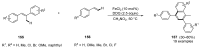


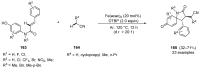






References
-
- Varun B.V., Dhineshkumar J., Bettadapur K.R., Siddaraju Y., Alagiri K., Prabhu K.R. Recent advancements in dehydrogenative cross coupling reactions for C-C bond formation. Tetrahedron Lett. 2017;58:803–824. doi: 10.1016/j.tetlet.2017.01.035. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

