Drug Screening of Flavonoids as Potential VEGF Inhibitors Through Computational Docking and Cell Models
- PMID: 39860127
- PMCID: PMC11767819
- DOI: 10.3390/molecules30020257
Drug Screening of Flavonoids as Potential VEGF Inhibitors Through Computational Docking and Cell Models
Abstract
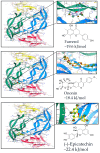
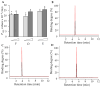
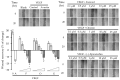
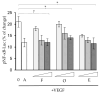
Vascular endothelial growth factor (VEGF), also known as VEGF-A, has been linked to various diseases, such as wet age-related macular degeneration (wAMD) and cancer. Even though there are VEGF inhibitors that are currently commercially available in clinical applications, severe adverse effects have been associated with these treatments. There is still a need to develop novel VEGF-based therapeutics against these VEGF-related diseases. Here, we established a series of VEGF-based computational docking analyses and cell models, such as a wound healing assay in HaCaT cells and an evaluation of NF-κB performance in macrophages, to screen a large library of flavonoid-type phytochemicals. Three flavonoids, namely, farrerol, ononin and (-)-epicatechin, were shown to express binding affinities to VEGF protein and inhibit VEGF-mediated biological activities. The investigation evidently suggested that the three flavonoids above could be considered potential anti-VEGF agents for the following drug development against VEGF-mediated diseases.
Keywords: VEGF inhibitor; angiogenesis; computational docking; drug screening; flavonoids.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

